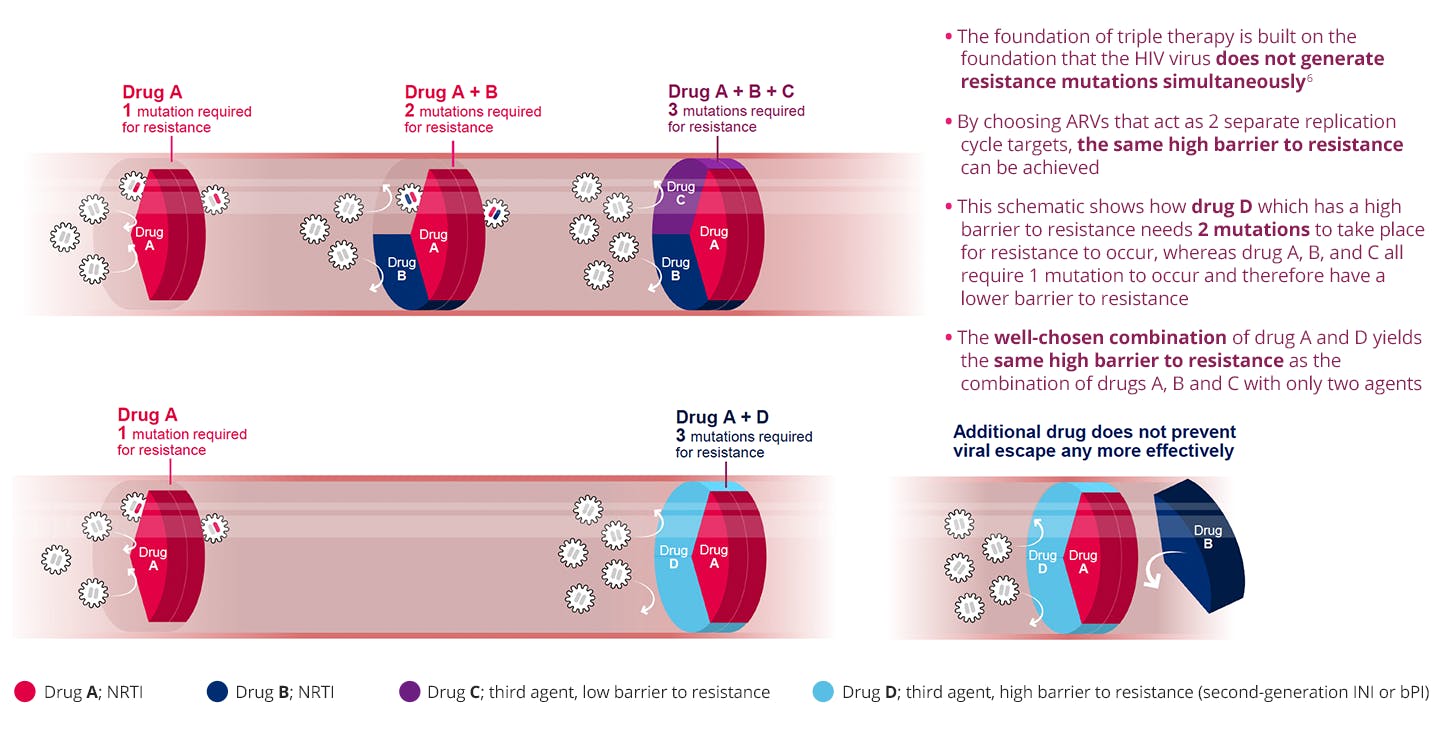
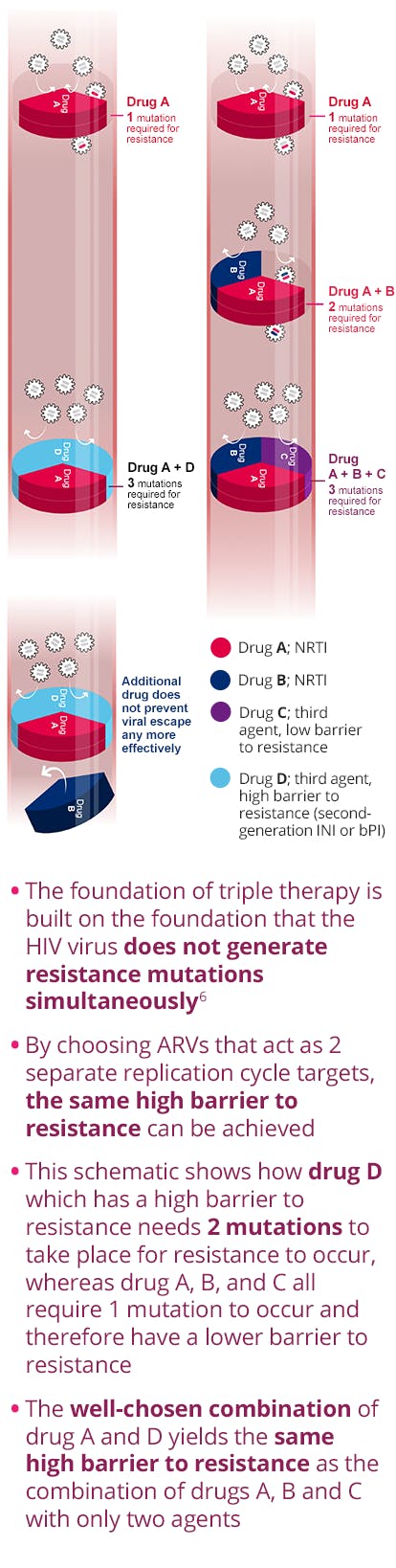
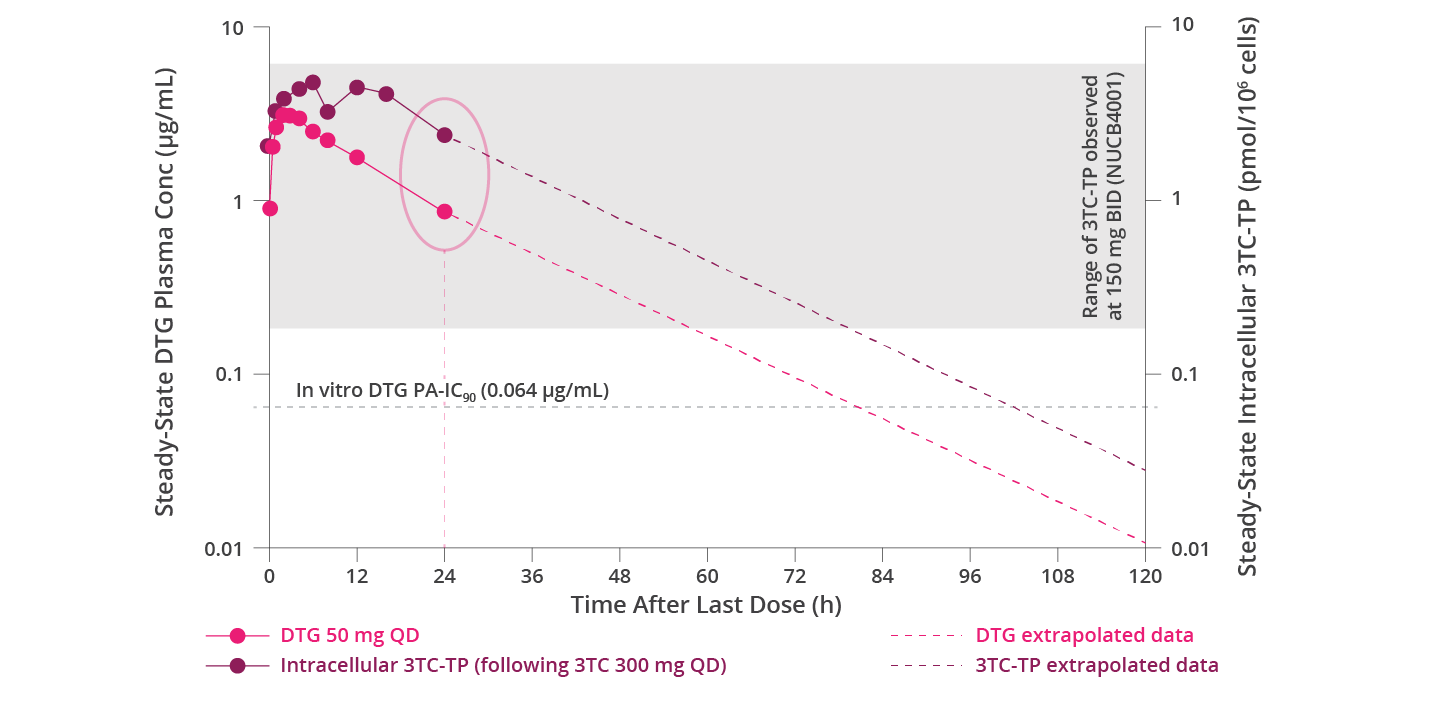
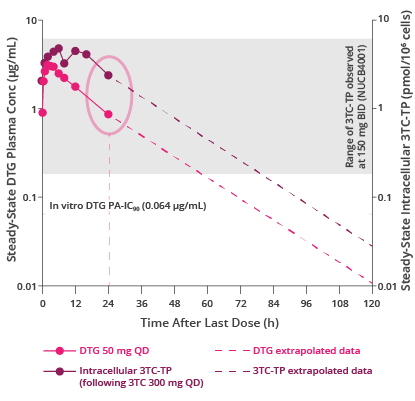
TP=triphosphate; PA=protein-adjusted.
Want to hear from the experts, including past webinar recordings?
References:
- Clutter DS, Jordan MR, Bertagnolio S, Shafer RW. HIV-1 drug resistance and resistance testing. Infect Genet Evol. 2016;46:292-307. doi:10.1016/j.meegid.2016.08.031
- Tseng A, Seet J, Phillips EJ. The evolution of three decades of antiretroviral therapy: challenges, triumphs and the promise of the future. Br J Clin Pharmacol. 2014;79(2):182-194. doi:10.1111/bcp.12403
- Hirsch MS, Conway B, D’Aquila RT, et al. Antiretroviral drug resistance testing in adults with HIV infection: implications for clinical management. JAMA. 1998;279(24):1984-1991. doi:10.1001/jama.279.24.1984
- Perelson AS, Neumann AU, Markowitz M, Leonard JM, Ho DD. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science. 1996;271(5255):1582-1586. doi:10.1126/science.271.5255.1582
- Feng Q, Zhou A, Zou H, et al. Quadruple versus triple combination antiretroviral therapies for treatment naive people with HIV: systematic review and meta-analysis of randomised controlled trials. BMJ. 2019;366:l4179. doi:10.1136/bmj.l4179
- Perelson AS, Essunger P, Ho DD. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. AIDS. 1997;11(suppl A):S17-S24.
- Moore KHP, Barrett JE, Shaw S, et al. The pharmacokinetics of lamivudine phosphorylation in peripheral blood mononuclear cells from patients infected with HIV-1. AIDS. 1999;13(16):2239-2250. doi:10.1097/00002030-199911120-00006
- Yuen GJ, Lou Y, Bumgarner NF, et al. Equivalent steady-state pharmacokinetics of lamivudine in plasma and lamivudine triphosphate within cells following administration of lamivudine at 300 milligrams once daily and 150 milligrams twice daily. Antimicrob Agents Chemother. 2004;48(1):176-182. doi:10.1128/AAC.48.1.176-182.2004
- Min S, Sloan L, DeJesus E, et al. Antiviral activity, safety, and pharmacokinetics/pharmacodynamics of dolutegravir as 10-day monotherapy in HIV-1 infected adults. AIDS. 2011;25(14):1737-1745. doi:10.1097/QAD.0b013e32834a1dd9
- TIVICAY. US Package insert. ViiV Healthcare group of companies; 2021.
- Cahn P, Sierra Madero J, Arribas JR, et al. Three-year durable efficacy of dolutegravir plus lamivudine in antiretroviral therapy-naïve adults with HIV-1 infection. AIDS. 2022;36(1):39-48. doi:10.1097/QAD.0000000000003070
- Osiyemi O, De Wit S, Ajana F, et al. Efficacy and safety of switching to dolutegravir/lamivudine (DTG/3TC) versus continuing a tenofovir alafenamide-based 3- or 4-drug regimen for maintenance of virologic suppression in adults living with HIV-1: results through week 144 from the phase 3, non-inferiority TANGO randomized trial. Clin Infect Dis. 2022;ciac036 and suppl 1-18. doi:10.1093/cid/ciac036
October 2022 PM-GB-DLL-WCNT-220005
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.


