FOSTEMSAVIR▼CHARACTERISTICS
Learn more about the key attributes of fostemsavir (RUKOBIA) based on the Phase III BRIGHTE data.
Story of Fostemsavir
The development of fostemsvir has been many years in the making. Here we describe its journey from conception to development. phase clinicals, and EMA and FDA approval
MECHANISM OF ACTION
Entry of the HIV into a target cell occurs through a process of 3 main steps. HIV entry inhibitors are varied in their point of action. Here we detail where the different entry inhibitors act and if they are pre attachment of post attachment. Fostemsavir has a new mechanism of action that is able tobind onto gp120, to prevent HIV attachment and entry.
The Three Stages of HIV Cell Entry
Entry of the HIV-1 virus into a target cell occurs through 3 main steps
HIV-1 cell entry and lifecycle
An overview of the HIV-1 lifecycle, from cell entry to the production of new virions
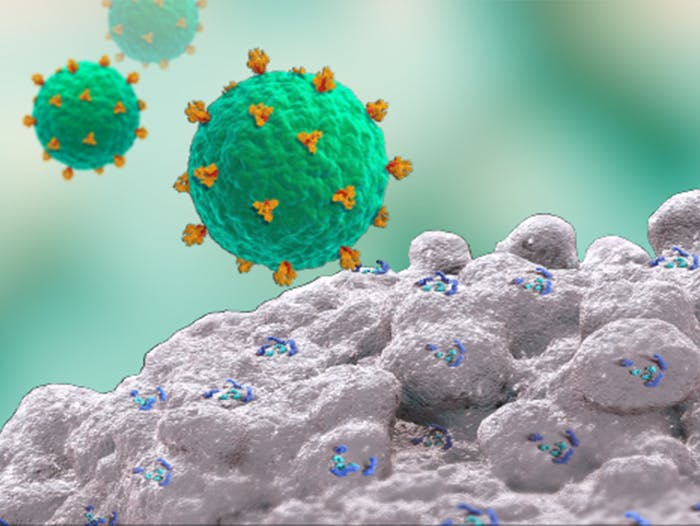
Fostemsavir's Mechanism of Action
Fostemsavir has a unique mechanism of action that is able to bind viral gp120 preventing HIV-1 attachment and entry
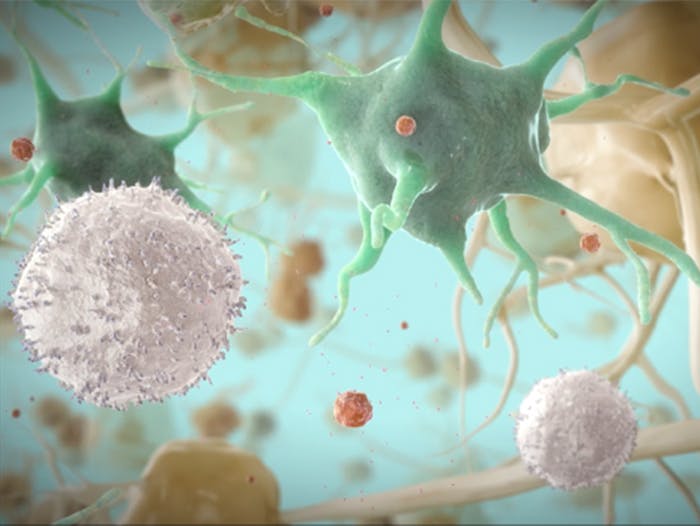
Elimination of HIV Following Fostemsavir Action
After fostemsavir blocks HIV-1 attachment to target cells, the virus of informacion tor products remains in the extracellular space where it can be eliminated by the and host immune responses
240wk BRIGHTE Study Results
An overview of outcomes at 240 Week from the BRIGHTE Phase Ill study of fostemsavir in heavily treatment experienced patients.
Building an Antiretroviral OBR with Fostemsavir in a clinic setting
An overview of important considerations for building an optimized background therapy with fostemsavir in a clinic environment
NP-GBL-FST-WCNT-230002 November 2023
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.