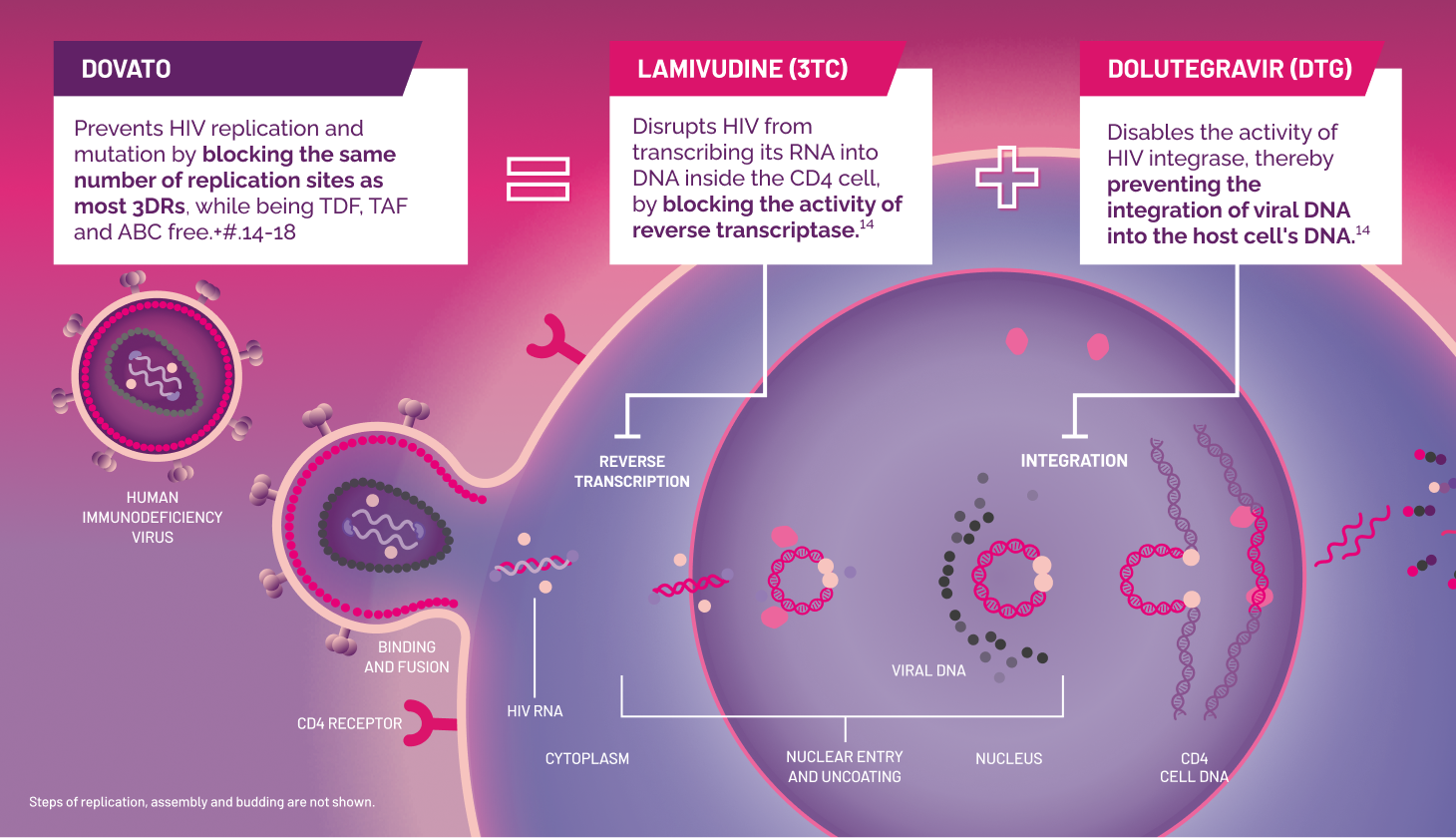
DOVATO is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and adolescents above 12 years of age weighing at least 40kg, with no known or suspected resistance to the integrase inhibitor class, or lamivudine.
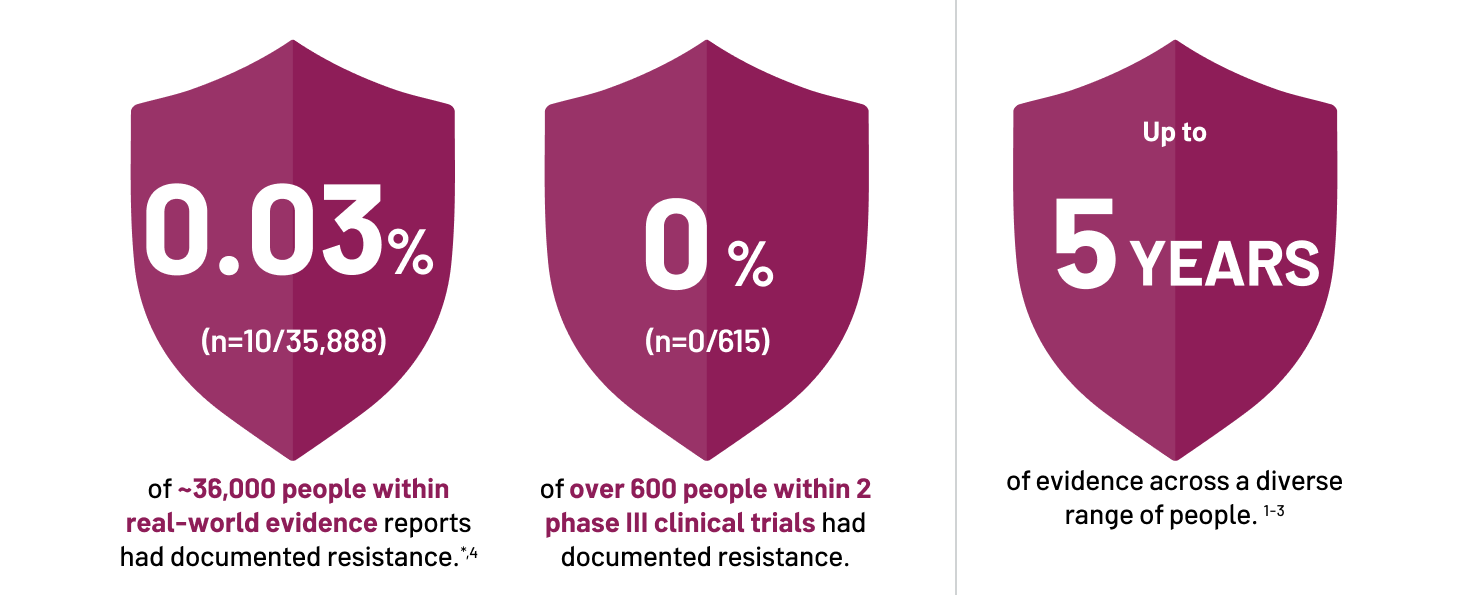
CONFIDENCE IN DOVATO'S HIGH BARRIER TO RESISTANCE ACROSS TREATMENT SETTINGS
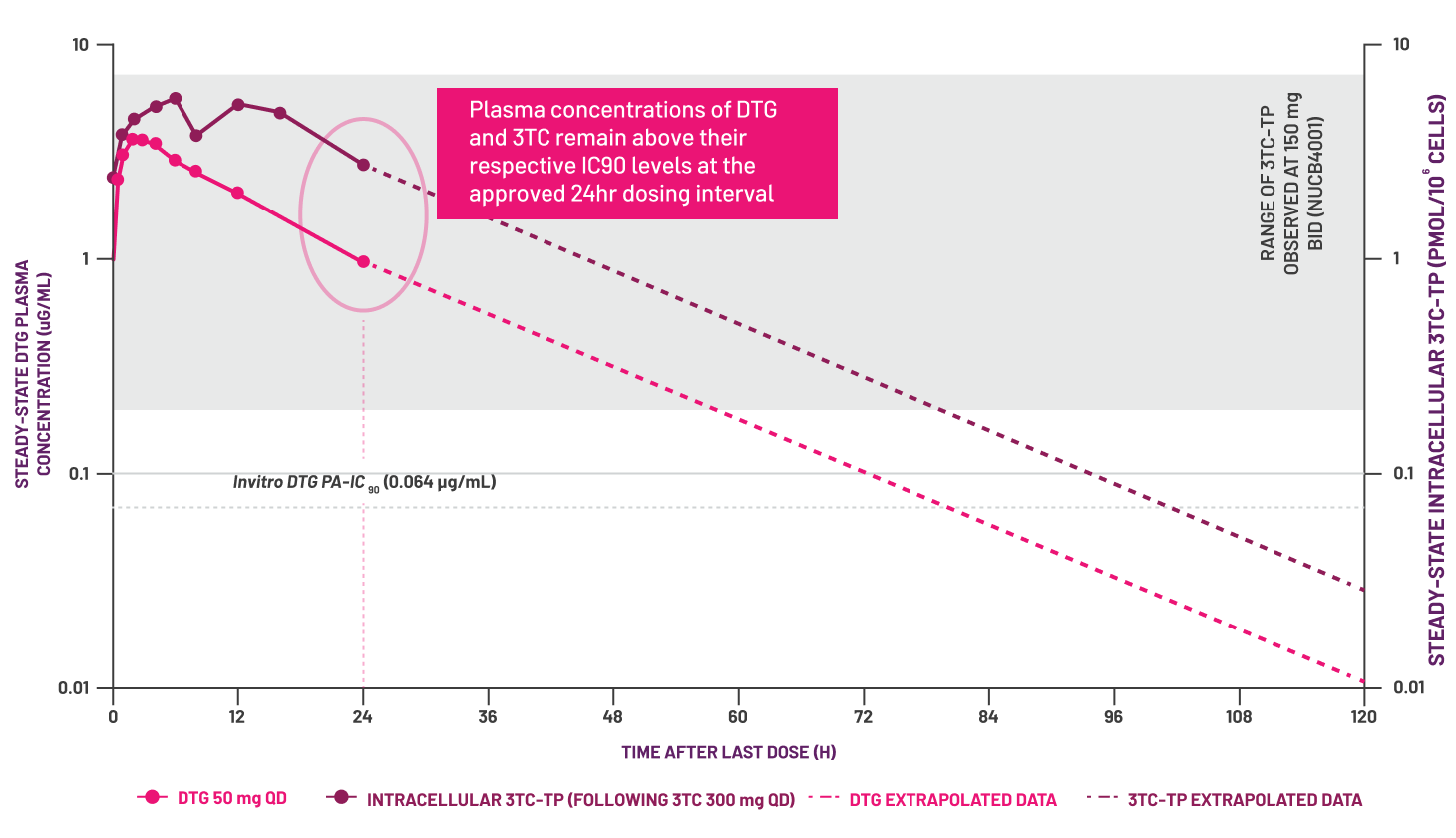
Adapted from Boffito M et al, 2020.[12]
Efficacy of DOVATO across treatment settings
See data from the relevant randomised controlled trials and real-world evidence
3DR, 3-drug regimen; 3TC, lamivudine; ABC, abacavir; BID, twice daily; CVW, confirmed virologic withdrawal; DNA, deoxyribonucleic acid; DTG, dolutegravir; FDA, United States Food and Drug Administration; FTC, emtricitabine; ITT-E, intention-to-treat exposed; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PA-IC90, protein-adjusted 90% inhibitory concentration; PK, pharmacokinetic; QD, once daily; RNA, ribonucleic acid; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate; TP, triphosphate; XTC, emtricitabine or lamivudine.
* Data extracted from a systematic literature review of DTG+3TC real-world evidence. Overlap between cohorts cannot be fully excluded.[4]
† The reported rate reflects the sum-total of resistance cases calculated from GEMINI I and II (n=1/716, through 144 Weeks), STAT (n=0/131, through 52 Weeks), and D2ARLING (n=0/106, through 24 Weeks). [5–7]
‡ GEMINI I and II are two identical 148-Week, phase III, randomised, double-blind, multicentre, parallel-group, non-inferiority, controlled clinical trials testing the efficacy of DOVATO in treatment-naïve patients. Participants with screening HIV-1 RNA ≤500,000 copies/mL were randomised 1:1 to once-daily DOVATO (n=716, pooled) or DTG + TDF/FTC (n=717, pooled). The primary endpoint of each GEMINI study was the proportion of participants with plasma HIV-1 RNA <50 copies/mL at Week 48 (ITT-E population, snapshot algorithm). In GEMINI, 0 cases of resistance were reported among participants who met protocol-defined confirmed virologic withdrawal (CVW). Participants met CVW criteria if a second and consecutive HIV-1 RNA value met any of the following: decrease from baseline in plasma HIV-1 RNA <1 log10 copies/mL, unless HIV-1 RNA <200 copies/mL, by Week 12; confirmed plasma HIV-1 RNA ≥200 copies/mL at or after Week 24; or plasma HIV-1 RNA ≥200 copies/mL after previous confirmed viral suppression to HIV-1 RNA <200 copies/mL.DTG 50 mg + 3TC 300 mg was used in the GEMINI studies.[5,13]
§ STAT is a phase IIIb, open-label, 48-week, single-arm pilot study evaluating the feasibility, efficacy, and safety of DTG/3TC in 131 newly diagnosed HIV-1 infected adults as a first-line regimen. The primary endpoint was the proportion of participants with plasma HIV-1 RNA <50 copies/mL at Week 24.[6]
|| D2ARLING is a randomised, open-label, phase IV study designed to assess the efficacy and safety of DTG/3TC in treatment-naïve people with HIV with no available baseline HIV-1 resistance testing. Participants were randomised in a 1:1 ratio to receive DTG/3TC (n=106) or DTG + TDF/XTC (n=108). The primary endpoint was the proportion of participants with plasma HIV-1 RNA <50 copies/mL at Week 48.[7]
¶ The reported rate reflects the sum-total of resistance cases calculated from TANGO (n=0/369, through 196 Weeks) and SALSA (n=0/246, through 48 Weeks).[8,9]
# TANGO is a randomised, open-label, trial testing the efficacy of DOVATO in virologically suppressed patients. Participants were randomised in a 1:1 ratio to receive DOVATO (n=369) or continue with TAF-containing regimens (n=372) for up to 200 weeks. At Week 148, 298 of those on TAF-based regimens switched to DOVATO. The primary efficacy endpoint was the proportion of subjects with plasma HIV-1 RNA ≥50 copies/mL (virologic non-response) as per the FDA Snapshot category at Week 48 (adjusted for randomisation stratification factor).[8,13]
††SALSA is a phase III, randomised, open-label, non-inferiority clinical trial evaluating the efficacy and safety of switching to DTG/3TC compared with continuing current antiretroviral regimens in virologically suppressed adults with HIV. Eligible participants were randomised 1:1 to switch to once-daily DTG/3TC (n=246) or continue current antiretroviral regimens (n=247). The primary endpoint was the proportion of subjects with plasma HIV-1 RNA ≥50 copies/mL at Week 48 (ITT-E population, snapshot algorithm).[9]
‡‡Most NRTI-based 3DRs.[14–16]
- Maggiolo F et al. BMC Infect Dis 2022; 22(1): 782.
- Ciccullo A et al. JAIDS 2021; 88(3): 234–237.
- Taramasso L et al. AIDS Patient Care STDS 2021; 35(9): 342–353.
- ViiV Healthcare. Data on File. REF-237004. 2024.
- Cahn P et al. AIDS 2022; 36(1): 39–48.
- Rolle C et al. Open Forum Infect Dis 2023; 10(3): 1–9.
- Cordova E et al. Poster presented at 12th IAS Conference on HIV Science. 23–26 July 2023. Brisbane, Australia. TUPEB02.
- De Wit S et. J Acquir Immune Defic Syndr 2024. doi: 10.1097/QAI.0000000000003395. [Epub ahead of print].
- Llibre J et al. Clin Infect Dis 2023; 76(4): 720–729.
- Rolle C et al. Poster presented ID Week. 11–15 October 2023. Boston, Massachusetts, USA. 1603.
- Ryan P, et al. AIDS 2024. OAB3606LB
- Boffito M et al. AIDS Res Hum Retroviruses 2020; 36(1): 13–18.
- Min S et al. Antimicrob Agents Chemother 2010; 54(1): 254–258.
- DOVATO (dolutegravir/lamivudine) Summary of Product Characteristics (SmPC)
- Tseng A et al. Br J Clin Pharmacol. 2014; 79(2): 182–194.
- Feng Q et al. BMJ 2019; 366: l4179.
- Rousseau F et al. J Infect Dis 2003; 188(11): 1652–1658.
- Perelson A et al. Science. 1996; 271(5255): 1582–1586.
PM-GB-DLL-WCNT-240005. September 2024
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.