DOVATO is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and adolescents above 12 years of age weighing at least 40kg, with no known or suspected resistance to the integrase inhibitor class, or lamivudine.
Efficacy outcomes have been evaluated in:
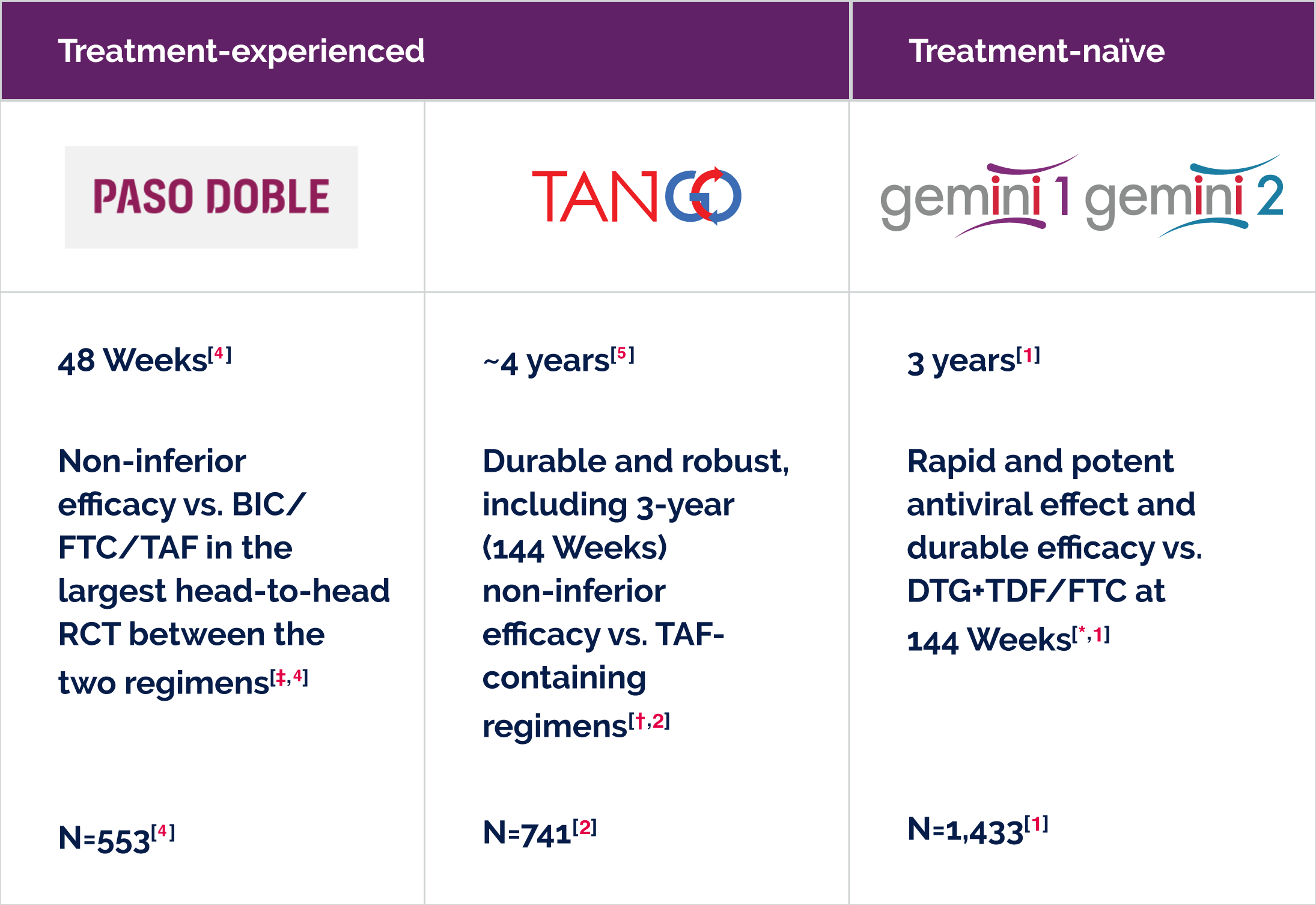
Treatment-naïve people living with HIV, so you can confidently prescribe DOVATO from the start.[1,12–15] |
Treatment-experienced people living with HIV, so you can switch to DOVATO whilst maintaining stable viral suppression.[2,5,8,16–20] |
|---|---|
DOVATO has proven efficacy in a diverse subset of treatment-naïve people living with HIV who have: |
Efficacy in treatment-experienced people living with HIV:
|
A well-established safety profile[3]
BIC, bictegravir; FTC, emtricitabine; DTG, dolutegravir; NRTI, nucleoside/nucleotide reverse transcriptase inhibitors; TAF, tenofovir alafenamide; TDF, tenofovir disoproxil fumarate.
* DTG 50 mg + 3TC 300 mg was used in the GEMINI studies. Primary endpoint: proportion of participants with plasma HIV-1 RNA <50 copies/mL at 48 Weeks in the intention-to-treat-exposed (ITT-E) population.[1]
† Primary endpoint: proportion of participants with plasma HIV-1 RNA ≥50 copies/mL at 48 Weeks using the FDA Snapshot algorithm the ITT-E population.[2]
‡ Primary endpoint: proportion of participants with plasma HIV-1 RNA ≥50 copies/mL at 48 weeks in the ITT-E population.[4]
§ Potential overlap between cohorts cannot be ruled out. Discrete cohorts are those included in the total number of people treated with DTG + 3TC in real-world.[6]
|| Results extracted from a pooled analysis of 48-Week data from TANGO and SALSA.[21]
- Cahn P et al. AIDS 2022; 36(1): 39–48.
- Osiyemi O et al. Clin Infect Dis 2022; 75(6): 975–986.
- DOVATO (dolutegravir/lamivudine) Summary of Product Characteristics (SmPC)
- Ryan P, et al. AIDS 2024. OAB3606LB
- De Wit S et. J Acquir Immune Defic Syndr 2024. doi: 10.1097/QAI.0000000000003395.
- ViiV Healthcare. Data on File. REF-237004. 2024.
- Rolle C et al. Open Forum Infect Dis 2023; 10(3): 1–9.
- Llibre J et al. Clin Infect Dis 2023; 76(4): 720–729.
- Maggiolo F et al. BMC Infect Dis 2022; 22(1): 782.
- Ciccullo A et al. JAIDS 2021; 88(3): 234–237.
- Taramasso L et al. AIDS Patient Care STDS 2021; 35(9): 342–353.
- Eron J et al. Slides presented at HIV DART and Emerging Viruses. 27–29 November 2018. Miami, Florida, USA. OP7.
- Pulido F et al. Poster presented at HIV Glasgow. 23–26 October 2022. Virtual and Glasgow, UK. P059.
- Kuretski J et al. Poster presented at IDWeek. 19–23 October 2022. Virtual and Washington, DC, USA. 1279.
- Cordova E et al. Poster presented at the 12th IAS Conference on HIV Science. 23–26 July 2023. Brisbane, Australia. TUPEB02.
- Rolle C et al. Poster presented at ID Week. 11–15 October 2023. Boston, Massachusetts, USA. 1603.
- Cheng C et al. Poster presented at the 19th European AIDS Conference. 18–21 October 2023. Warsaw, Poland. e.P.A.051.
- Gan L et al. Infect Dis Ther 2023; 12(11): 2581–2593.
- Knobel H et al. HIV Res Clin Pract 2023; 24(1): 2239564.
- Farinacci D et al. Poster presented at the 19th European AIDS Conference. 18–21 October 2023. Warsaw, Poland. e.P.A.038.
- Walmsley S, et al. AIDS Res Ther 2024; 21(1): 17.
PM-GB-DLL-WCNT-240004. September 2024
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.