DOVATO is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and adolescents above 12 years of age weighing at least 40kg, with no known or suspected resistance to the integrase inhibitor class, or lamivudine.


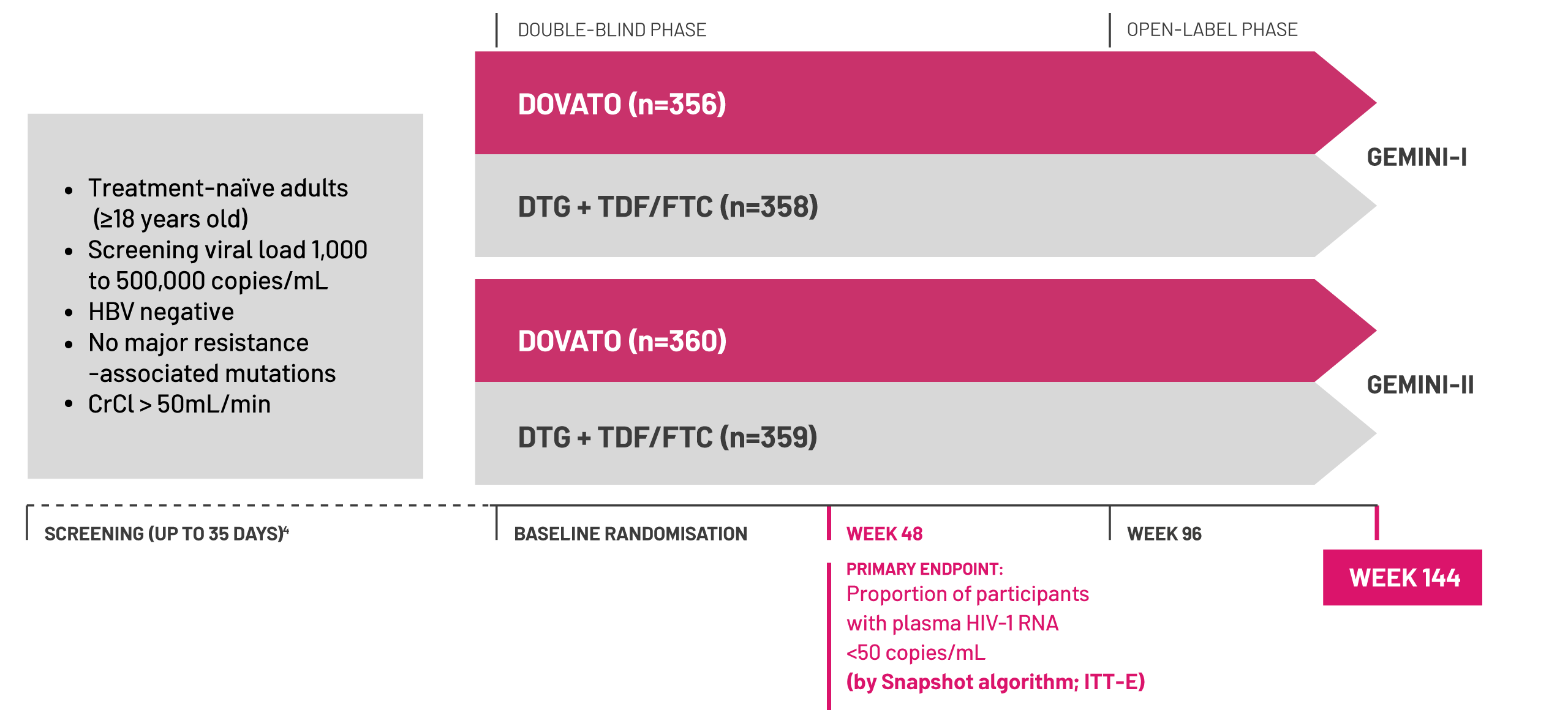
Adapted from Cahn P et al, 2022.[1]
Spotlight on the baseline viral loads of the participants included in the GEMINI I and II studies
of participants on DOVATO had baseline viral loads >100,000 copies/mL[1]
of participants in both arms had viral loads ≥500,000 copies/mL at baseline after screening[1]


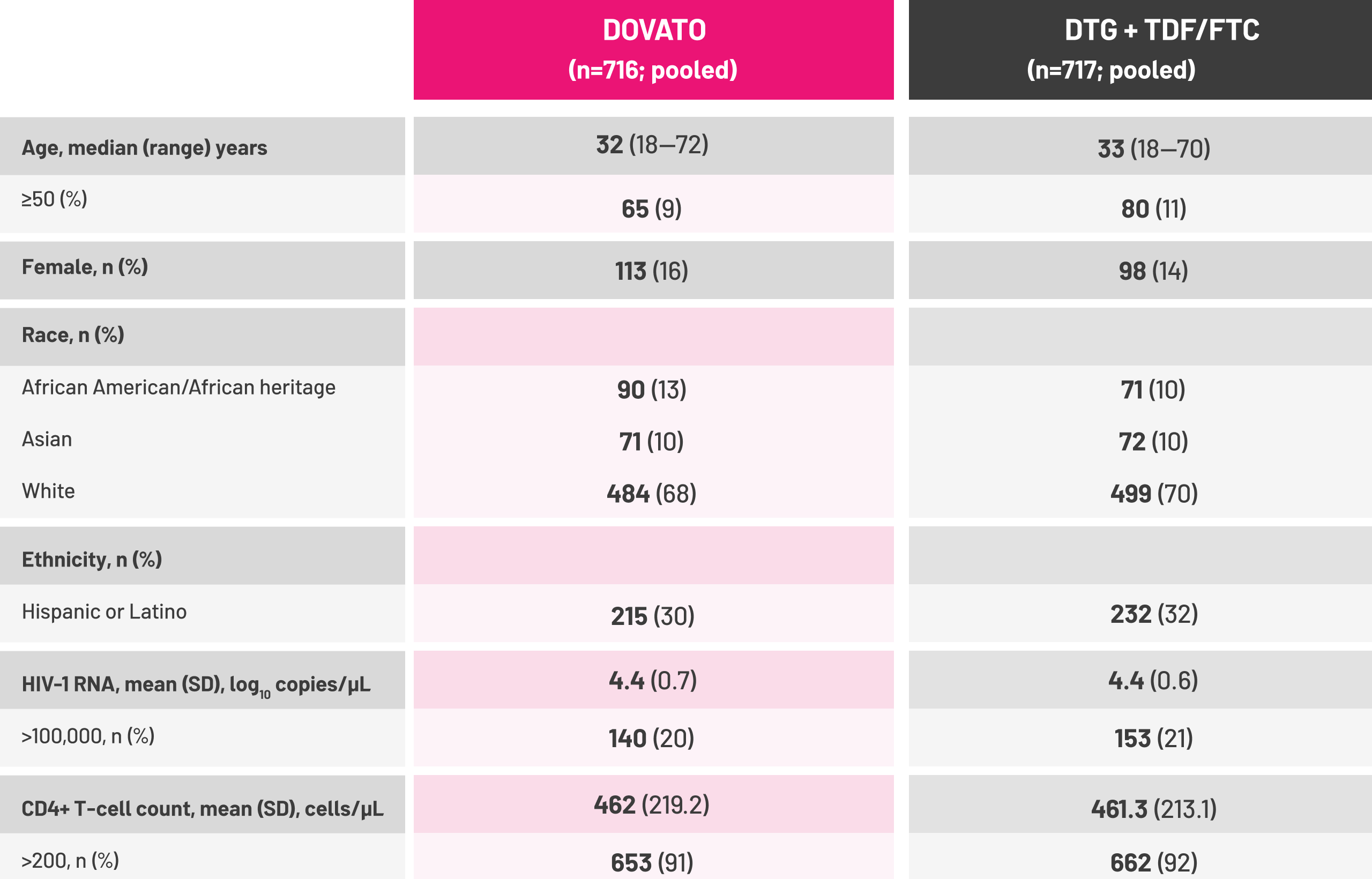
Adapted from Eron J et al, 2018.[5]

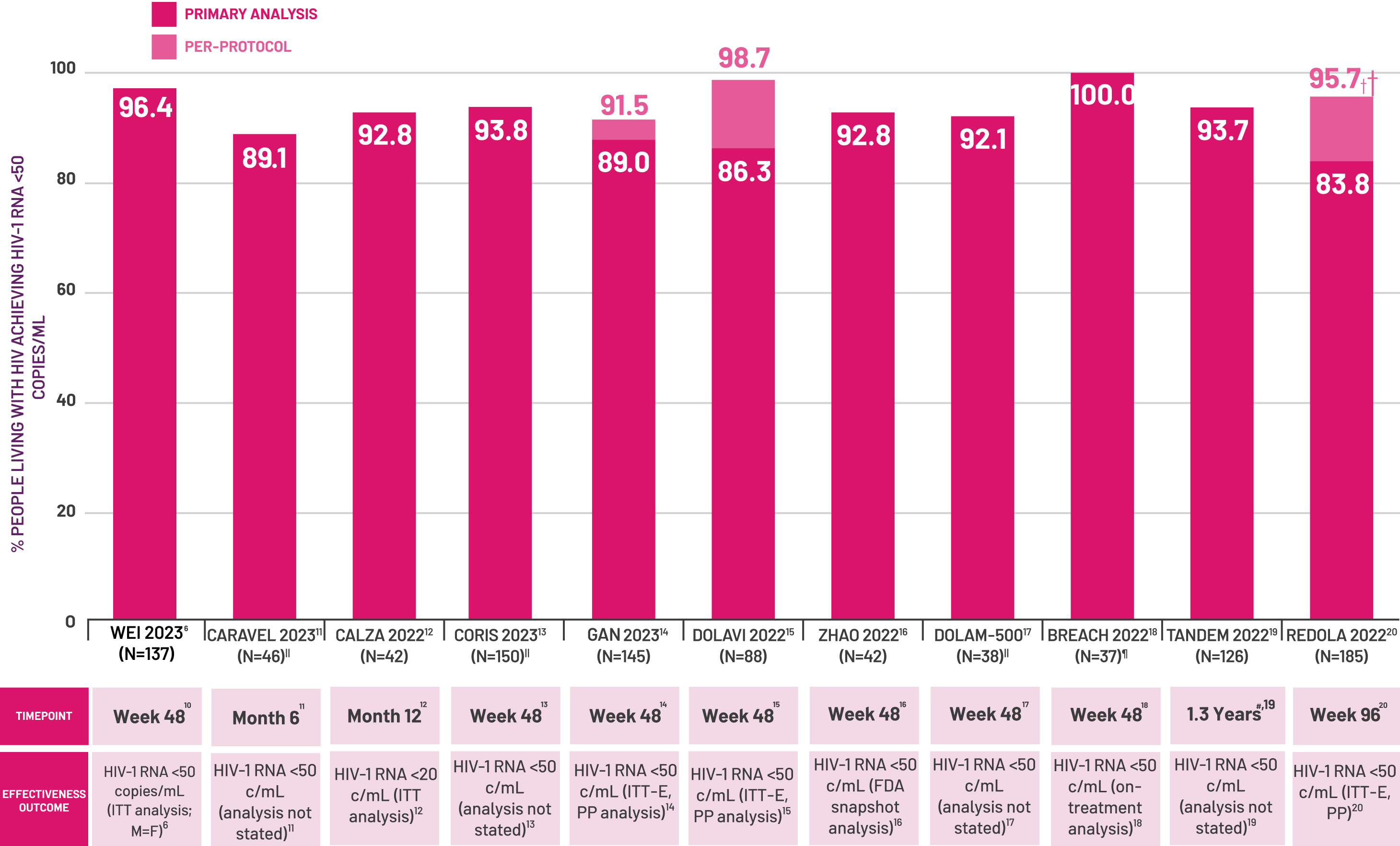
Adapted from a systematic literature review of DTG+3TC from real-life cohorts including Wei Y et al. 2023; Philibert P et al, 2023, Calza L et al 2022, Suarez-Garcia I et al, 2023, Long H et al, 2023, Hidalgo-Tenorio C et al, 2022, Zhao F et al, 2022, Inan A et al, 2023, Nasreddine R et al, 2023, Schneider S et al, 2022, Pulido F et al, 2022.[6–16]


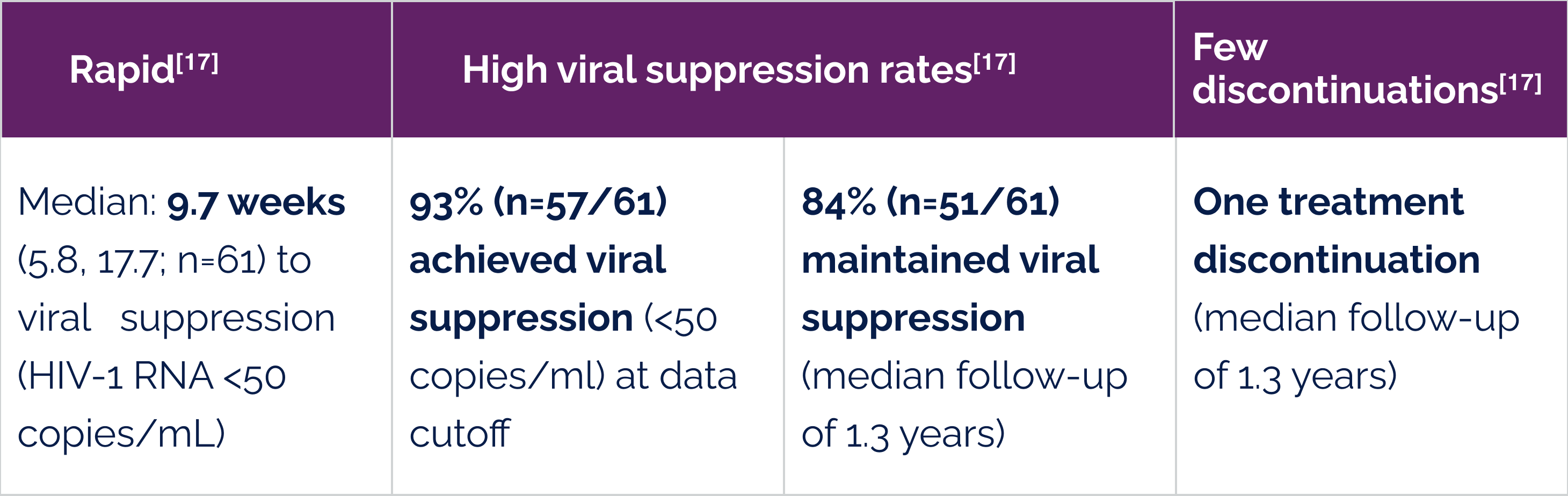
Effectiveness further supported in a small subset of people living with HIV with high baseline viral loads[18]
(n=8/9)
of those with baseline HIV-1 RNA 100,000–250,000 copies/mL achieved viral suppression.[18]
(n=6/7)
of those with baseline HIV-1 RNA >250,000 copies/mL achieved viral suppression.[18]
3DR, 3-drug regimen; 3TC, lamivudine; bDRT, baseline drug resistance testing; BL, baseline; CI, confidence interval; CVF, confirmed virological failure; DTG, dolutegravir; FTC, emtricitabine; FU, follow up; HBV, hepatitis B virus; IQR, interquartile range; ITT-E, intention-to-treat exposed; MEX, missing equals excluded; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; PP, per-protocol analysis; RNA, ribonucleic acid; RPV, rilpivirine; SD, standard deviation; T&T, test and treat; TDF, tenofovir disoproxil fumarate; XTC, emtricitabine or lamivudine.
*Once-daily DTG 50 mg + 3TC 300 mg used in the GEMINI studies.[1]
‡ Graph includes discrete cohorts reporting applicable effectiveness outcomes for ≥30 treatment-naïve people receiving DTG+3TC; reported effectiveness outcomes vary between studies; potential overlap between cohorts cannot be ruled out.[6–16]
§ Viral suppression defined as HIV-RNA <50 copies/mL or 50-200 copies/mL with subsequent HIV-RNA <50 copies/mL in the effectiveness set.[6]
|| People excluded due to missing data or not completing FU: CARAVEL n=10, CoRIS n=251, DOLAM-500 n=18.[7,9,13]
¶ N=37 treatment-naive people at BL, n at Week 48.[14]
# Median time over which treatment-naïve people became suppressed was 10.4 weeks.[15]
†† 162 people were included in the PP analysis.[16]
- Cahn P et al. AIDS 2022; 36(1): 39–48.
- Osiyemi O et al. Clin Infect Dis 2022; 75(6): 975–986.
- DOVATO (dolutegravir/lamivudine) Summary of Product Characteristics (SmPC)
- Cahn P et al. Lancet 2019; 393(10167): 143–155.
- Eron J et al. Presented at HIV DART and Emerging Viruses. 27–29 November 2018. Miami, USA. OP7.
- Wei Y et al. Chin Med J 2023; 136: 2677-2685
- Philibert P et al. Poster presented at 19th European IDS Conference. 18–21 October 2023. Warsaw, Poland. e.P.A.014.
- Calza L et al. J Acquir Immune Defic Syndr 2022; 91(4): e9–e11.
- Suárez-García I. J Antimicrob Chemother 2023; 78: 1423–1432.
- Long H et al. Poster presented at 19th European AIDS Conference. 18–21 October 2023. Warsaw, Poland. e.P.A.058.
- Hidalgo-Tenorio C et al. Viruses 2022; 14(524): 1–11.
- Zhao F et al. J Acquir Immune Defi c Syndr 2022; 91(S1): S16–S19.
- Inan A et al. Poster presented at 19th European AIDS Conference. 18–21 October 2023. Warsaw, Poland. 992.
- Nasreddine R et al. HIV Med 2023; 24(3): 267–278.
- Schneider S et al. Poster presented at the 24th International AIDS Conference. 29 July–2 August 2022. Virtual and Montreal, Canada. EPB147.
- Pulido F et al. Poster presented at HIV Glasgow. 23–26 October 2022. Glasgow, UK. P059.
- Kuretski J et al. Poster presented at IDWeek. 19–23 October 2022. Washington, USA. 1279.
- Benson P et al. Poster presented at IDWeek. 19–23 October 2022. Washington, USA. 1278.
- Maggiolo F et al. BMC Infect Dis 2022; 22(1): 782.
- Ciccullo A et al. JIADS 2021; 1—14.
- Taramasso L et al. AIDS Patient Care STDs 2021; 35(9): 342—353.
PM-GB-DLL-WCNT-240004. September 2024
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.


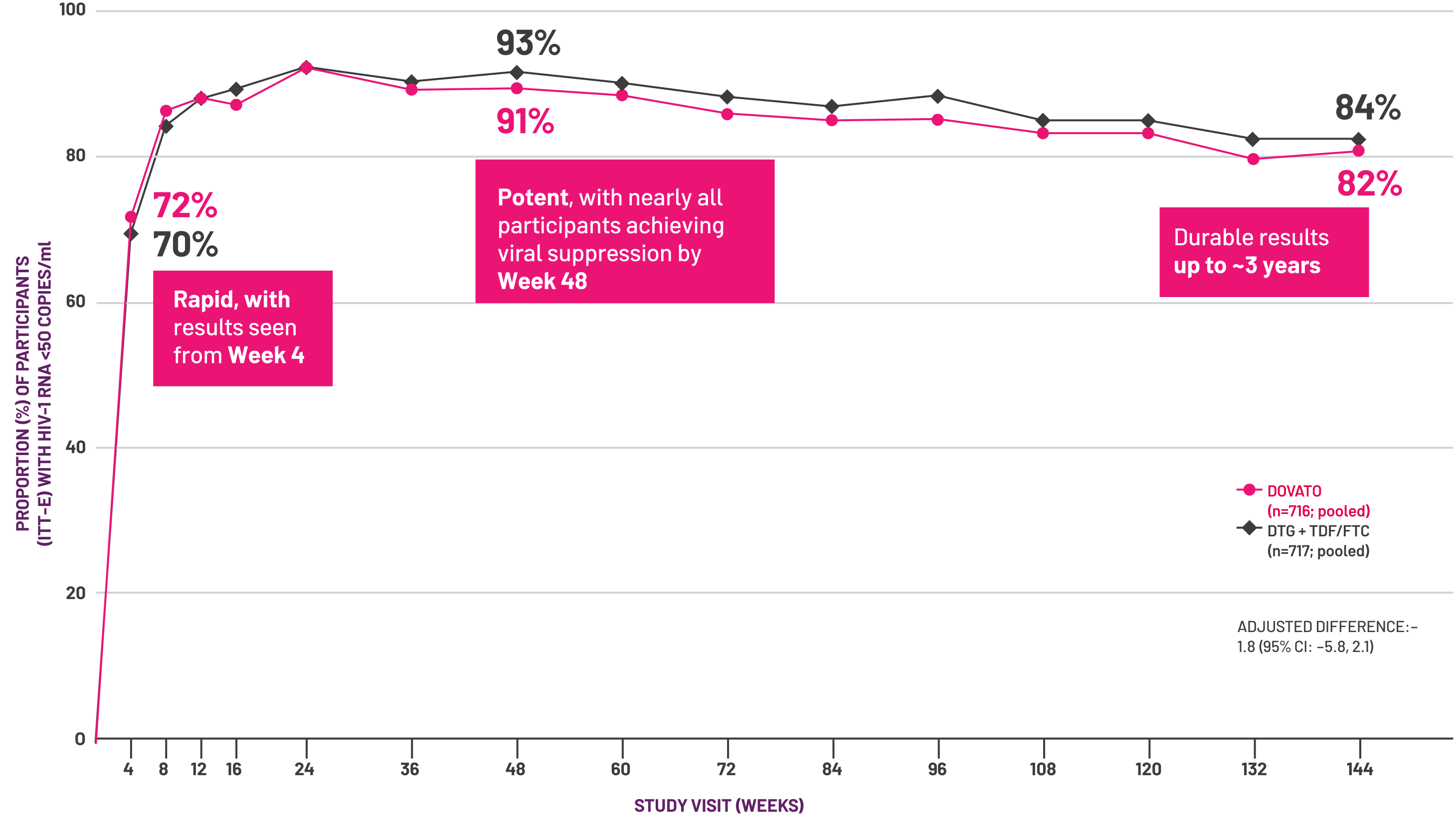
![Adapted from Eron J et al, 2018.[5]](/content/dam/cf-viiv/viivexchange/en_GB/dovato-2024/viral-load-decline-graphs.png)