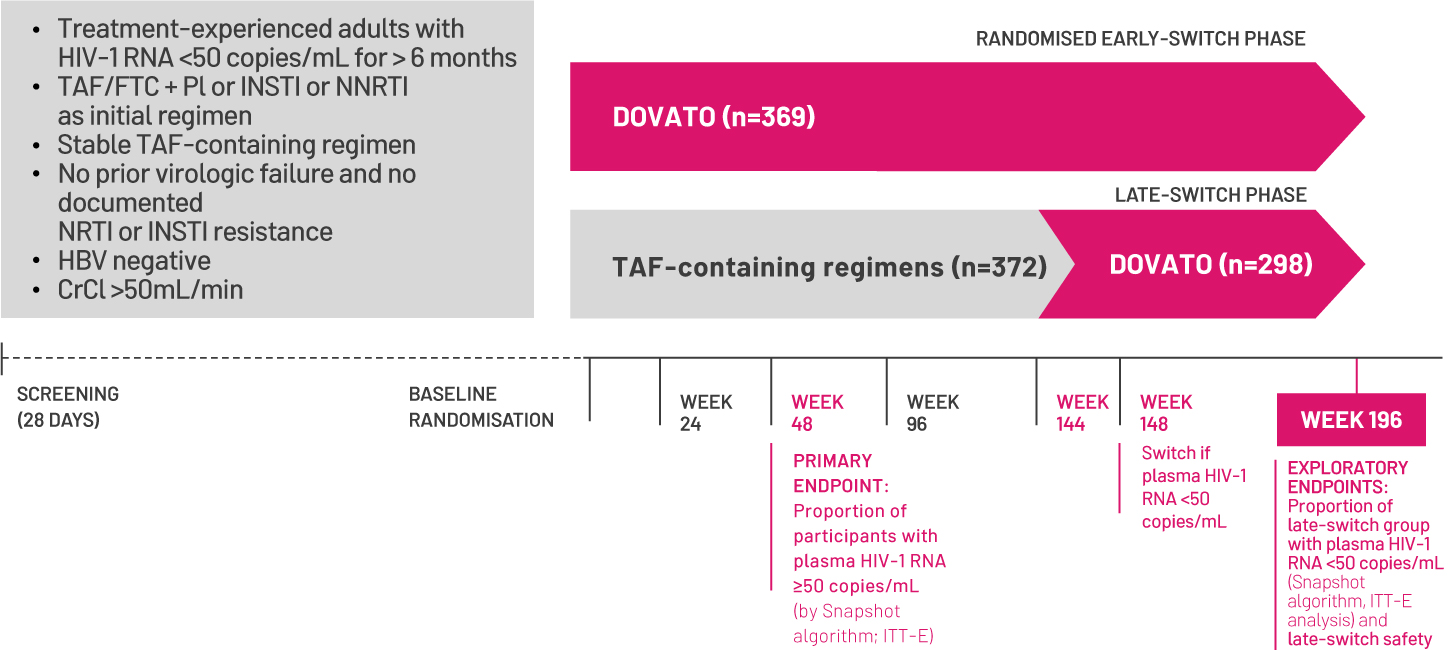
DOVATO is indicated for the treatment of human immunodeficiency virus type 1 (HIV-1) infection in adults and adolescents above 12 years of age weighing at least 40kg, with no known or suspected resistance to the integrase inhibitor class, or lamivudine.

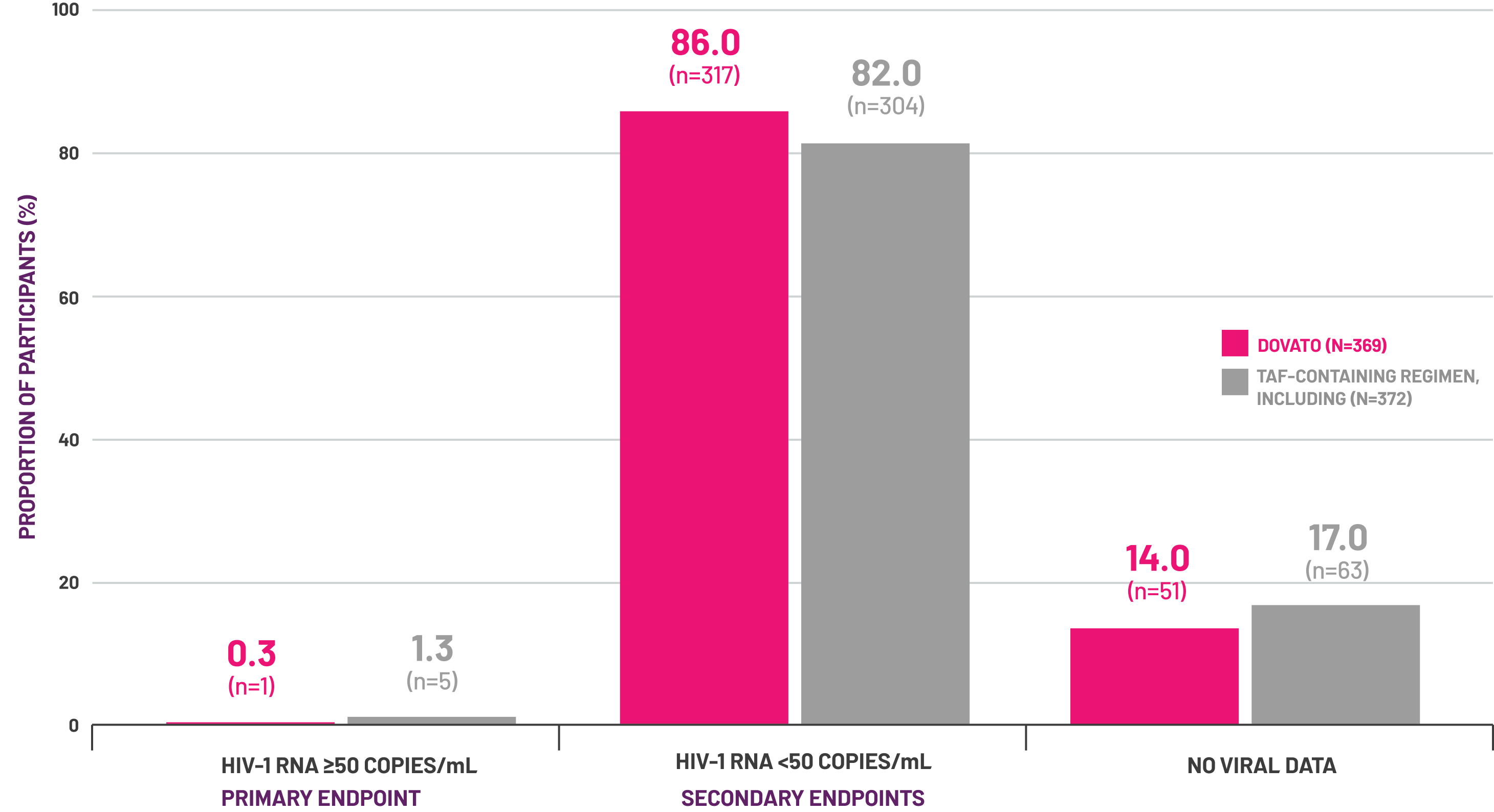
~4-year data (196 Weeks) confirms durable viral suppression in early switch participants with:[4]
(n=306/369)
of participants on DOVATO maintained HIV-1 RNA <50 copies/mL (Snapshot, ITT-E analysis)
(n=306/369)
of participants on DOVATO maintained viral suppression (observed analysis)


Adapted from Walmsley S et al, 2024.[6]
3DR, 3-drug regimen; 3TC, lamivudine; 4DR, four-drug regimen; AE, adverse event; ARV, antiretroviral; ART, antiretroviral therapy; bDRV, boosted darunavir; BIC, bictegravir; CI, confidence interval; DTG, dolutegravir; FDA, FTC, emtricitabine; HBV, hepatitis B virus; HCV, hepatitis C virus; IQR, interquartile range; INSTI, integrase strand transfer inhibitors; ITT-E, intention-to-treat exposed; NNRTI, non-nucleoside reverse transcriptase inhibitors; NRTI, nucleoside/nucleotide reverse transcriptase inhibitor; ns, non-significant; PI, protease inhibitor; RNA, ribonucleic acid; RPV, rilpivirine; TAF, tenofovir alafenamide;
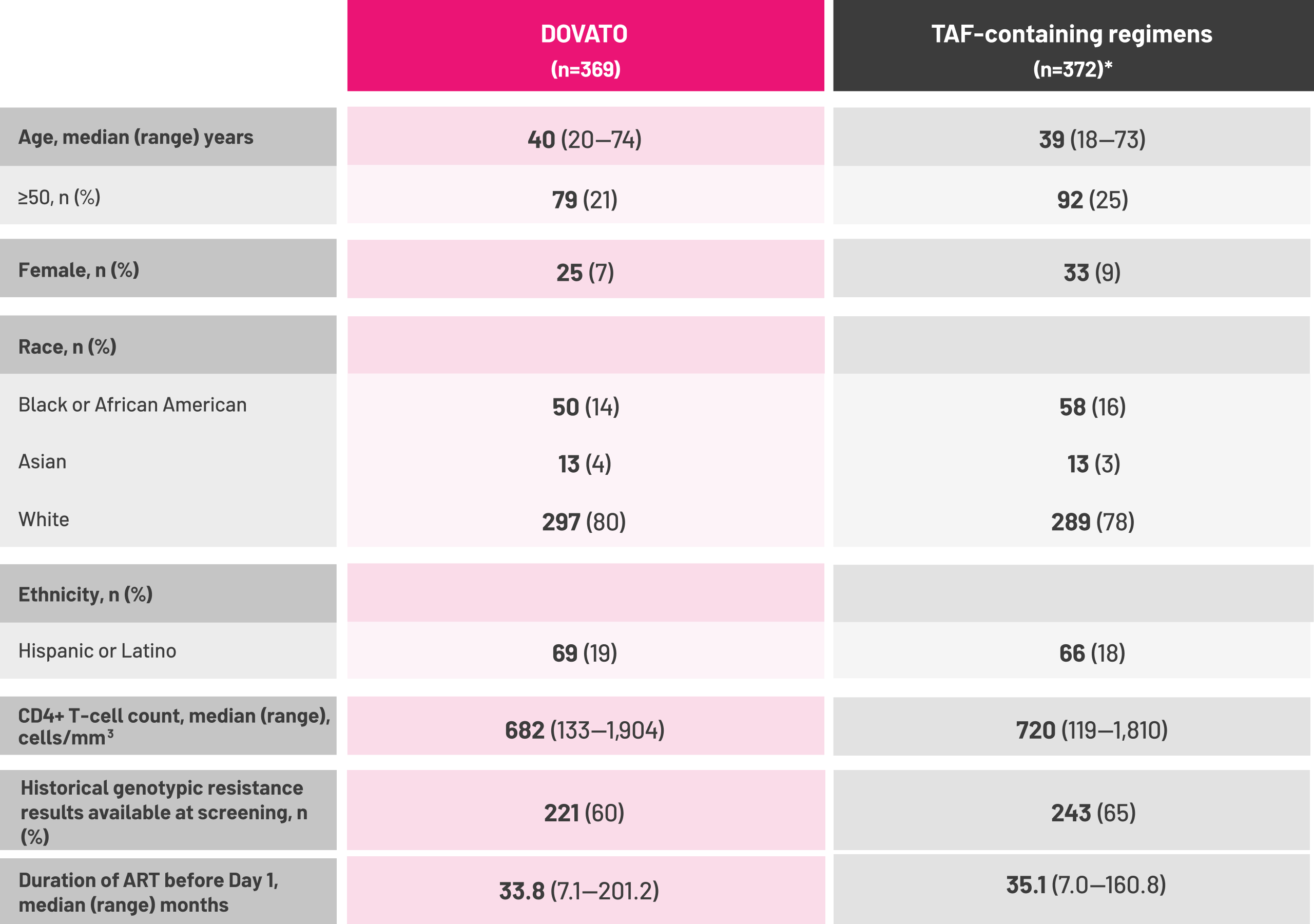
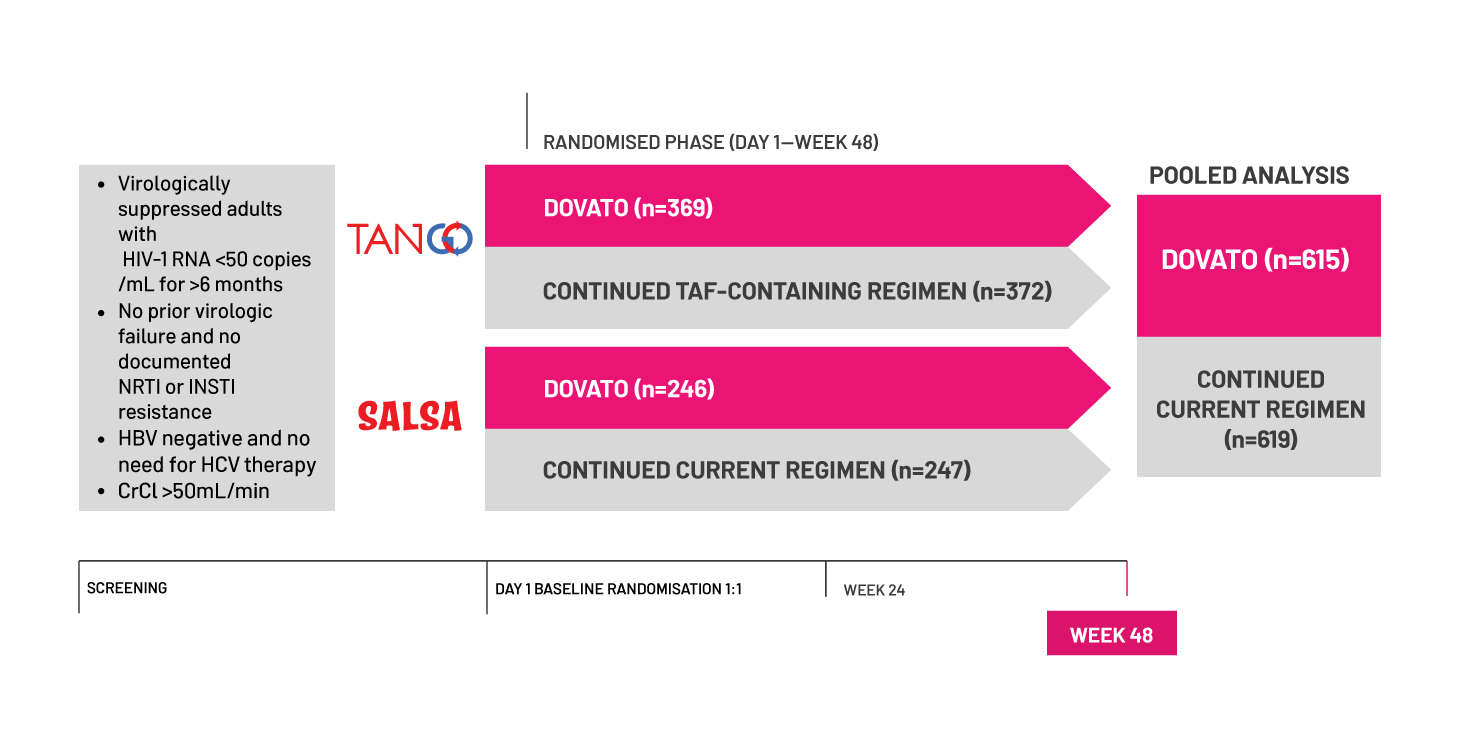
*Baseline third agent class included (DOVATO vs. TAF-containing regimens respectively): INSTI (n=289/369 vs. 296/372), EVG/c (n=243/369 vs. 249/372); NNRTI (n=51/369 vs. 48/372), RPV (n=43/369 vs. 45/372); PI (n=29/369 vs. 28/372), bDRV (n=25/369 vs. 27/372).[1]
†Historical resistance results (post hoc analysis) provided at screening were not recorded in the electronic case report form nor were they part of the locked database but are data on file that have been source verified and archived in the study trial master file.[1]
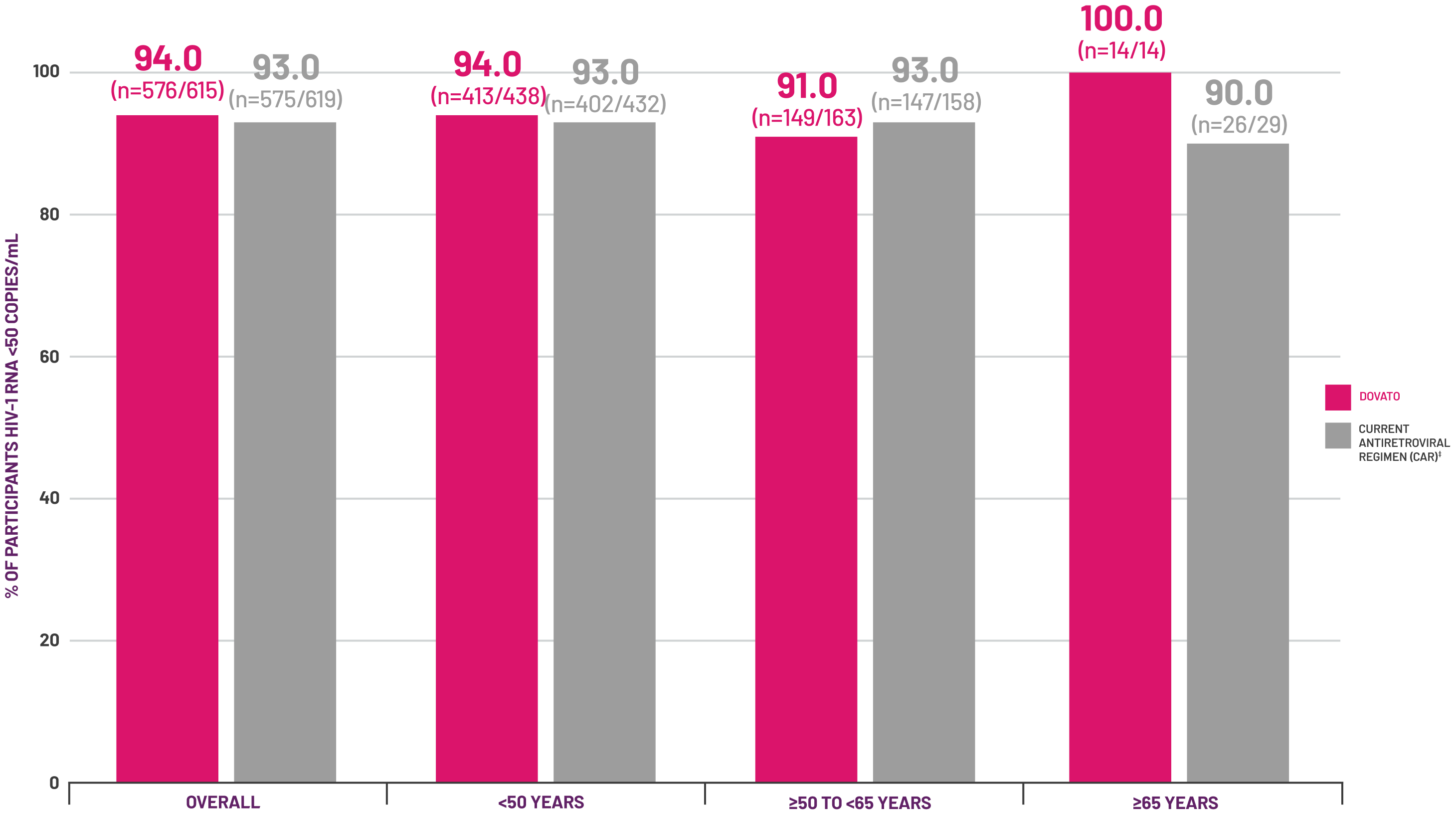
‡ Baseline NRTI for the CAR arm: TDF 18% (n=110/606), TAF 76% (n=462/606), ABC 6% (n=34/606).[6]
§ Baseline third agent class included (DOVATO vs. current antiretroviral regimen, respectively): INSTI (387/615 vs. 394/619), NNRTI (174/615 vs. 172/619), PI (54/615 vs. 53/619).[6]
- Osiyemi O et al. Clin Infect Dis 2022; 75(6): 975–986.
- DOVATO (dolutegravir/lamivudine) Summary of Product Characteristics (SmPC)
- Cahn P et al. AIDS 2022; 36(1): 39–48.
- De Wit S et. J Acquir Immune Defic Syndr 2024. doi: 10.1097/QAI.000000000000339. [Epub ahead of print].
- Van Wyk J et al. Clin Infect Dis 2020; 71(8): 1920–1929.
- Walmsley S, et al. AIDS Res Ther 2024; 21(1): 17.
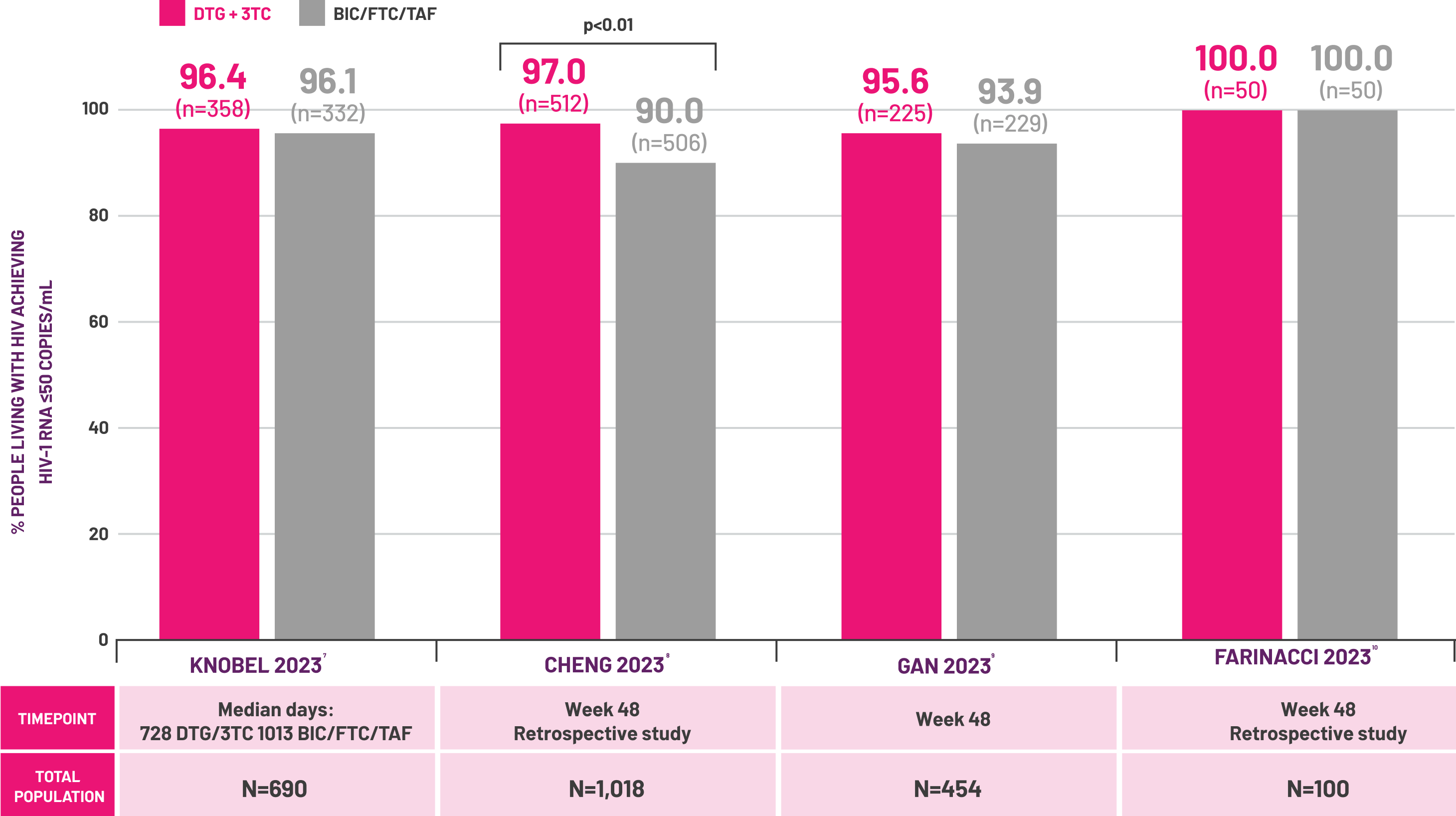
- Knobel H et al. HIV Res Clin Pract 2023; 24(1): 2239564.
- Cheng C et al. Poster presented at the 19th European AIDS Conference. 18–21 October 2023. Warsaw, Poland. e.P.A.051.
- Gan L et al. Infect Dis Ther 2023; 12(11): 2581–2593.
- Farinacci D et al. Poster presented at the 19th European AIDS Conference. 18–21 October 2023. Warsaw, Poland. e.P.A.038.
- Maggiolo F et al. BMC Infect Dis 2022; 22(1): 782.
- Ciccullo A et al. JIADS 2021; 1—14.
- Taramasso L et al. AIDS Patient Care STDs 2021; 35(9): 342—353.
- Rolle C et al. Open Forum Infect Dis 2023; 10(3): 1–9.
PM-GB-DLL-WCNT-240004. September 2024
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.