INFOGRAPHICS
Explore our summarised infographics.
Showing results for
DOVATO
Data from PASO DOBLE study from AIDS 2024
VOCABRIA + REKAMBYS
Key data evaluating the efficacy, safety, and tolerability of switching virologically suppressed (HIV-1 RNA ,50 copies/mL) adults to every 2-months Vocabria + Rekambys – versus continuing maintenance therapy with oral standard of care - in a sub-Saharan African population. The study was managed using a public health approach with viral load testing every 24 weeks.
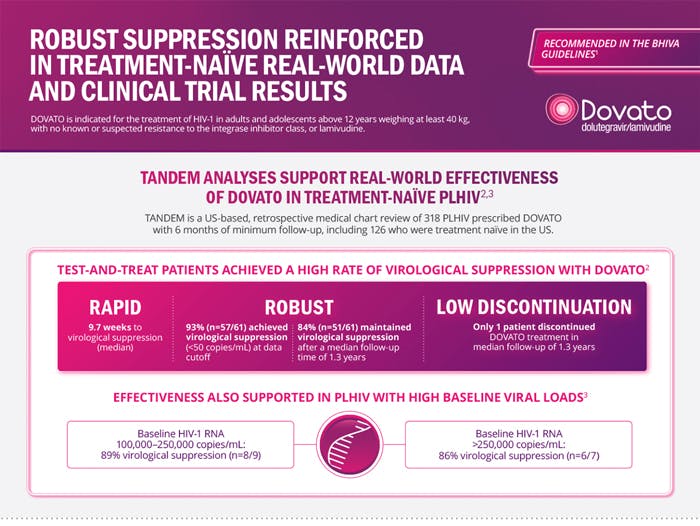
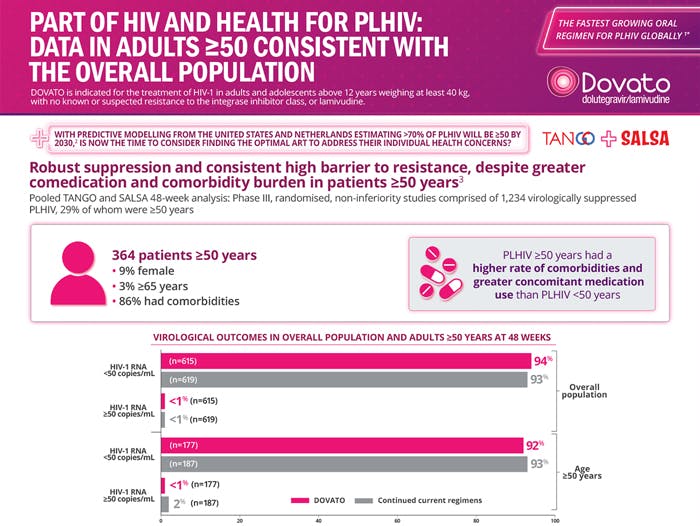
DOVATO
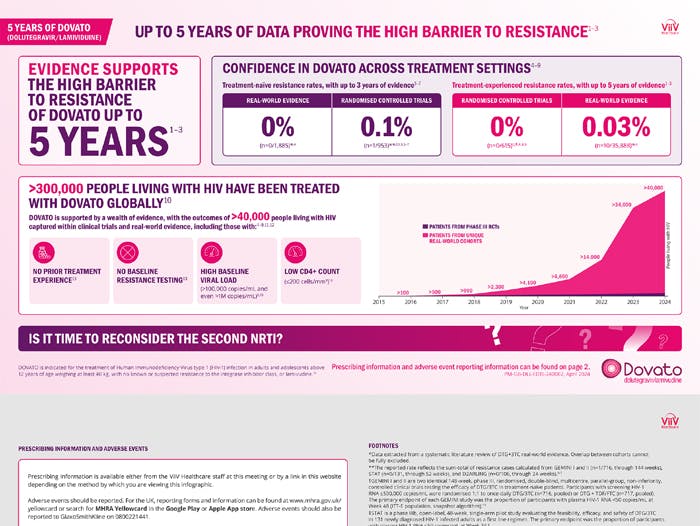
5 Years of Clinical and Real World Evidence Data
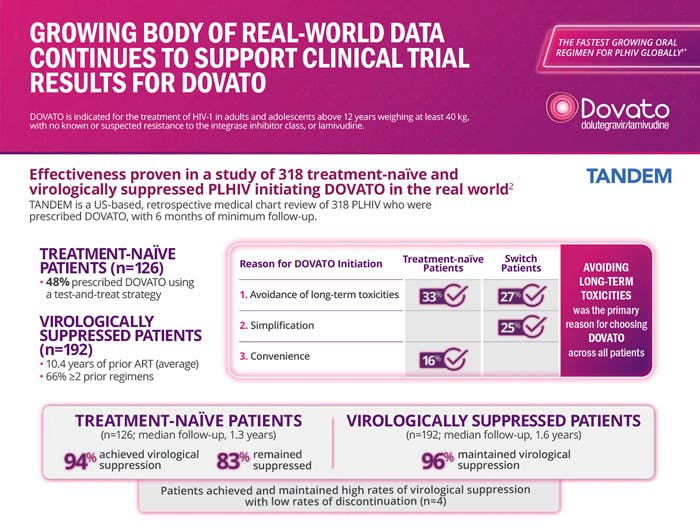
DOVATO
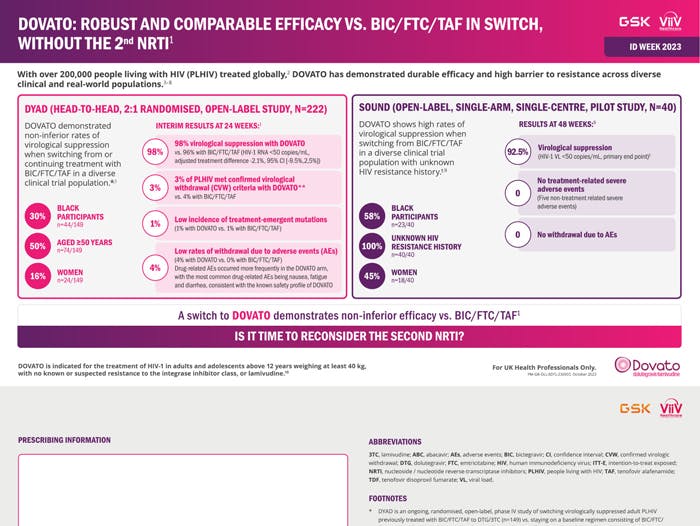
Data from the DYAD and SOUND study from ID Week 2023
DOVATO
24 week data from the D2ARLING study from IAS 2023
DOVATO
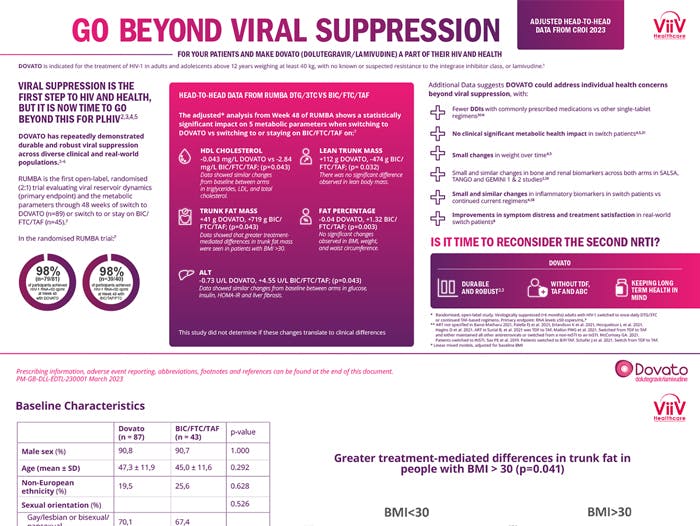
Head to Head data from RUMBA with DTG/3TC vs BIC/F
Other resources
PM-GB-HVX-WCNT-220003 | March 2024
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.