All People Living with HIV pictured and quoted in this website have been prescribed every-2-month VOCABRIA + REKAMBYS. They have given consent for the use of their images and words, and have received remuneration from ViiV Healthcare.
Indication: VOCABRIA injection is indicated, in combination with REKAMBYS injection, for the treatment of HIV-1 infection in adults who are virologically suppressed (HIV-1 RNA <50 copies/mL) on a stable antiretroviral regimen without present or past evidence of viral resistance to, and no prior virological failure with, agents of the NNRTI and INI class.[1,2]
INTRODUCING SOLAR
The first head-to-head switch study comparing every-2-month VOCABRIA + REKAMBYS to regular daily oral therapy[3]
SOLAR investigated the efficacy, safety and treatment experience of participants who were switched to every-2-month VOCABRIA + REKAMBYS (n=454) vs those who continued daily oral therapy with BIC/FTC/TAF (n=227).[3]
The SOLAR study endpoint was met (mITT- E population): Every-2-month VOCABRIA +REKAMBYS was non-inferior to daily oral therapy with BIC/FTC/TAF at month 12 (1.1% with plasma HIV-1 RNA ≥50 copies /ml [n=5/447] vs 0.4 [n=1/223], respectively)[3]

Patient preference
Preferred by 90% of participants over daily oral therapy with BIC/TFC/TAF[3]
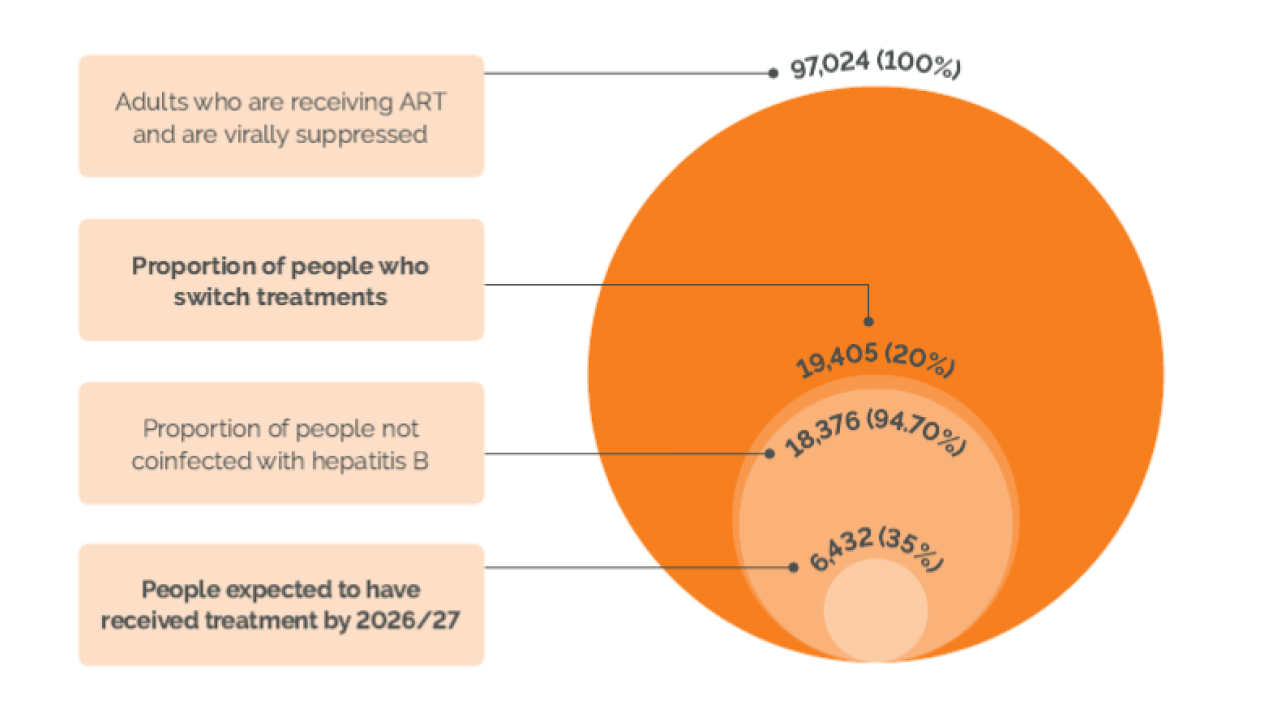
NICE state ‘clinical experts estimated that 20% would switch treatment due to issues with tolerability, toxicity, adherence (e.g. influenced by stigma, employment, travel, pill burden, “pill fatigue”) or malabsorption (e.g. due to comorbitites)’[5]
BIC/FTC/TAF=bictegravir/emtricitabine/tenofovir alafenamide; HIV=human immunodeficiency virus; HIV-1=human immunodeficiency virus type 1; INI=integrase inhibitor; NNRTI=non-nucleoside reverse transcriptase inhibitor; PLHIV=people living with HIV-1; RNA=ribonucleic acid.
References:
- VOCABRIA (cabotegravir) Summary of Product Characteristics. ViiV Healthcare.
- REKAMBYS (rilpivirine) Summary of Product Characteristics. Janssen.
- Ramgopal MN, Castagna A, Cazanave C, et al. SOLAR 12-Month Results – Randomized Switch Trial of CAB + RPV LA vs. Oral BIC/FTC/TAF. Presented at Conference on Retroviruses and Opportunistic Infections (CROI): February 19–22, 2023. REF-182990.
- Overton ET, Richmond G, Rizzardini G, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with human immunodeficiency virus 1 type 1 (HIV-1) infection: 152-week results from ATLAS-2M, a randomized, open-label, phase 3b, noninferiority study. Clin Infect Dis. 2023;ciad020. doi: 10.1093/cid/ciad020. Online ahead of print.
- NICE Resource impact report. Cabotegravir with rilpivirine for treating HIV-1 (Jan 2022). Available at https://www.nice.org.uk/guidance/ta757/resources/cabotegravir-with-rilpivirine-for-treating-hiv1-pdf-82611380476357. Accessed February 2024.
REKAMBYS (rilpivirine long-acting injection), including the trademark, is owned by the Janssen Pharmaceutical Companies and used under licence by the ViiV Healthcare group of companies. All other trademarks are owned by the ViiV Healthcare group.
PM-GB-CBR-WCNT-230001 | March 2024
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.