A RANGE OF RESOURCES TO HELP SUCCESSFULLY INTEGRATE EVERY-2-MONTH VOCABRIA + REKAMBYS INTO YOUR CLINIC
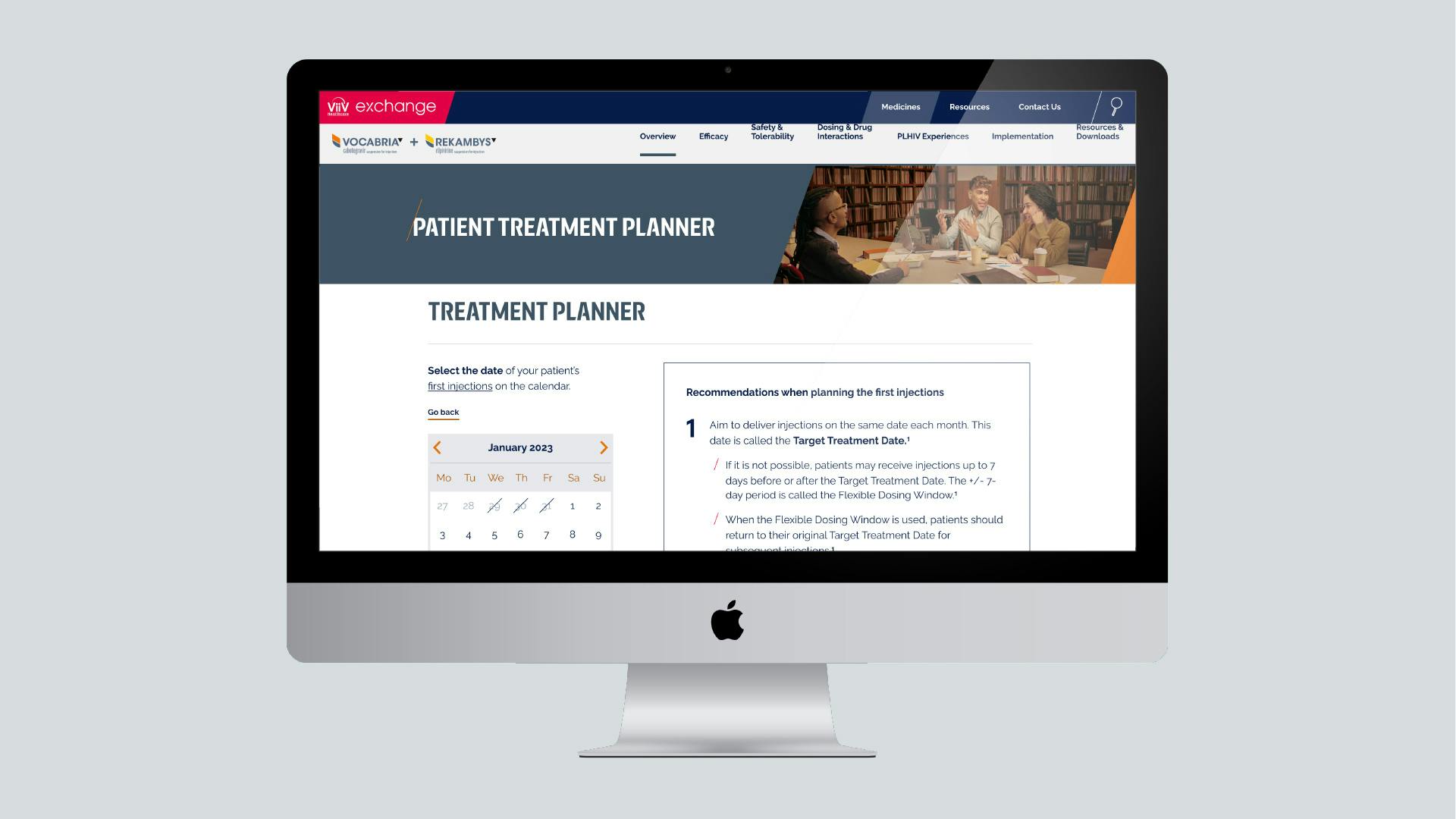
Treatment Planner
The Treatment Planner has been designed to make scheduling appointments for every-2-month VOCABRIA + REKAMBYS easier for you and your team.
Get started
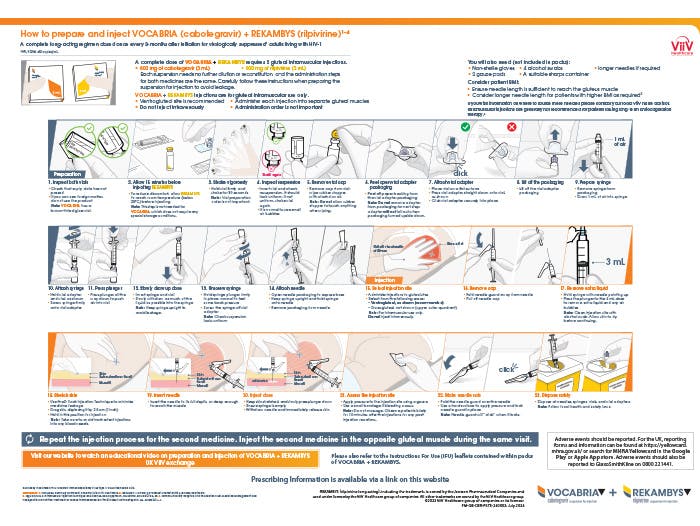
Preparation and injection tools
A summary of preparation and injection steps that can be viewed online.
PM-GB-CBR-WCNT-230005 | July 2024
To support you in planning and implementation in your clinic.
PM-GB-CBR-WCNT-230007 | July 2024

Administration Video
As the correct preparation and administration is important, we are here to help you. Watch this video to learn more.
PM-GB-CBR-WCNT-230006 | November 2024
To support you when deciding if a patient might be suitable for Vocabria and Rekambys.
PM-GB-CBR-WCNT-240006 | February 2024
To support you in the implementation of Long-acting Injectables (LAIs) into your clinical setting.
NP-GB-CBR-BKLT-250001 | February 2025
BIC/FTC/TAF=bictegravir/emtricitabine/tenofovir alafenamide; BMI=body mass index; FAQ=frequently asked question; HCP=healthcare professional; ID=identification; PLHIV=people living with HIV
REKAMBYS (rilpivirine long-acting injection), including the trademark, is owned by the Janssen Pharmaceutical Companies and used under license by the ViiV Healthcare group of companies. All other trademarks are owned by the ViiV Healthcare group.
PM-GB-CBR-WCNT-230004 | February 2025
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.