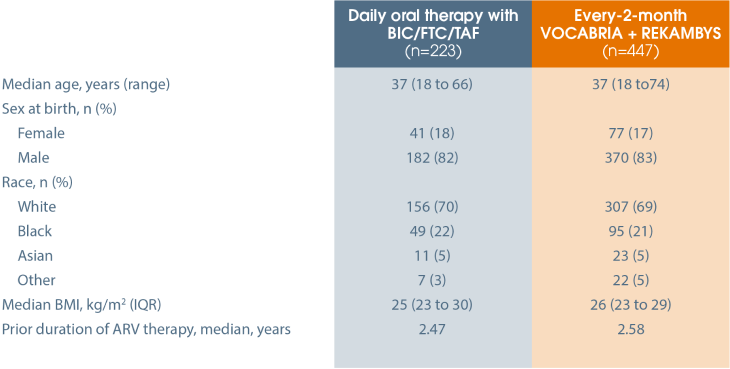SOLAR IS THE FIRST HEAD-TO-HEAD SWITCH STUDY COMPARING EVERY-2-MONTH VOCABRIA + REKAMBYS WITH REGULAR DAILY ORAL THERAPY[1]
In this phase IIIb, open label, non-inferiority study, virologically suppressed* adults with HIV-1 receiving daily oral therapy with BIC/FTC/TAF were randomised to one of three arms for the duration of the 12-month maintenance period*:
Due to protocol non-compliance, 11 subjects from one site were excluded from the intention-to-treat exposed (ITT-E) population, creating a modified intention-to-treat exposed (mITT-E) population. Efficacy is reported on a mITT-E analysis.[1]
SOLAR explored virological suppression, safety and tolerability, as well as patient treatment experiences with every-2-month VOCABRIA + REKAMBYS vs daily oral therapy with BIC/FTC/TAF[1]
The SOLAR study endpoint was met (mITT- E population): Every-2-month VOCABRIA +REKAMBYS was non-inferior to daily oral therapy with BIC/FTC/TAF at month 12 (1.1% with plasma HIV-1 RNA ≥50 copies /ml [n=5/447] vs 0.4 [n=1/223], respectively)[3]
Challenges revealed by SOLAR patients on daily oral therapy with BIC/FTC/TAF at baseline[1]
of participants on BIC/FTC/TAF (N=670) reported always or often experiencing at least one of the following psychological challenges:[1]

Worried about people unintentionally discovering their HIV status
Worried about forgetting to take their HIV medication
Felt that taking their HIV medication was an uncomfortable reminder of their HIV status
*Participants were suppressed for ≥6 months, virologic suppression defined as HIV-1 RNA <50 copies/mL and received BIC/FTC/TAF for ≥6 months prior to screening.[1]
†Cabotegravir injections, 600 mg; rilpivirine injections, 900 mg.[1]
‡CVF defined as 2 consecutive measurements of HIV-1 RNA ≥200 copies/mL.[1]
AE=adverse event; BIC/FTC/TAF=bictegravir/emtricitabine/tenofovir alafenamide; BMI=body mass index; CAB=cabotegravir; CVF=confirmed virologic failure; HBV=hepatitis B virus; HIV=human immunodeficiency virus; HIV-1=human immunodeficiency virus type 1; INI=integrase inhibitor; LA=long-acting; mITT-E=modified intention-to-treat exposed; NNRTI=non-nucleoside reverse transcriptase inhibitor; OLI=oral lead-in; PI=protease inhibitor; PLHIV=people living with HIV-1; RNA=ribonucleic acid; RPV=rilpivirine; SWI=start with injection.
References:
- Ramgopal MN, Castagna A, Cazanave C, et al. SOLAR 12-Month Results – Randomized Switch Trial of CAB + RPV LA vs. Oral BIC/FTC/TAF. Presented at Conference on Retroviruses and Opportunistic Infections (CROI): February 19–22, 2023. REF-182990.
- A Study to Evaluate Efficacy and Safety of Cabotegravir (CAB) Long Acting (LA) Plus (+) Rilpivirine (RPV) LA Versus BIKTARVY (BIK) in Participants With Human Immunodeficiency Virus (HIV)-1 Who Are Virologically Suppressed (SOLAR). 2022. www.clinicaltrials.gov/ct2/show/NCT04542070. REF-182394. Accessed January 30, 2023.
REKAMBYS (rilpivirine long-acting injection), including the trademark, is owned by the Janssen Pharmaceutical Companies and used under licence by the ViiV Healthcare group of companies. All other trademarks are owned by the ViiV Healthcare group.
PM-GB-CBR-WCNT-230001 | March 2024
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.