IT STARTS WITH
FINDING A REGIMEN THAT YOUR PATIENT PREFERS
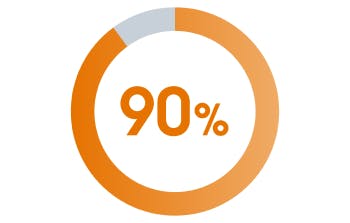
Every-2-month VOCABRIA + REKAMBYS was preferred by 9 out of 10 trial patients vs daily oral therapy with BIC/FTC/TAF[1]
Top five reasons selected by those who preferred every-2-month VOCABRIA + REKAMBYS in the SOLAR study (n=382):[1]
IT STARTS WITH
PROVEN EFFICACY
Every-2-month VOCABRIA + REKAMBYS was non-inferior to daily oral therapy with BIC/FTC/TAF[1]
Plasma HIV-1 RNA <50 copies/mL at Month 12 (modified intention-to-treat exposed [mITT-E])[1]
(secondary endpoint; 12% non‑inferiority margin)
The primary analysis was based on the modified intention-to-treat exposed (mITT-E) population (exclusion of one trial site for the non-compliance to protocol entry criteria)
The solar study endpoint was met (mITT- E population): Every-2-month VOCABRIA +REKAMBYS was non-inferior to daily oral therapy with BIC/FTC/TAF at month 12 (1.1% with plasma HIV-1 RNA ≥50 copies /ml [n=5/447] vs 0.4 [n=1/223], respectively)[3]
Low incidence of confirmed virologic failure (CVF) at Month 12[1]
As a prespecified secondary endpoint, patients who met the protocol-defined CVF criteria (ITT-E;3/681) were tested for emergent INSTI (cabotegravir) or NNRTI (rilpivirine) substitutions conferring resistance at month 12[1]
SOLAR CVF AND OVERALL REISISTANCE-ASSOCIATED MUTATIONS AT MONTH 12 (ITT-E)[1]
- Two (0.4%) participants receiving every-2-month VOCABRIA + REKAMBYS in the mITT-E population, and one additional participant† receiving every-2-month VOCABRIA + REKAMBYS in the ITT-E population, met the CVF criterion through Month 12[1]
- Two of the participants had on-treatment rilpivirine and/or INI resistance-associated mutations (genotyping for third participant failed at baseline)[1]
- All three participants with CVF and resistance re-suppressed on alternative antiretroviral drugs (ARVs)[1]
*Out of the 447 participants in the VOCABRIA + REKAMBYS arm, 425 responded; 90% preferred VOCABRIA + REKAMBYS; 5% reported preference for daily oral therapy with BIC/FTC/TAF; and the other 5% reported no preference.[1]
†Excluded from the mITT-E population due to critical findings related to non-compliance to protocol entry requirements at one study site.[1]
ARV=antiretroviral drug; BIC/FTC/TAF=bictegravir/emtricitabine/tenofovir alafenamide; CVF=confirmed virologic failure; HIV=human immunodeficiency virus; HIV-1=human immunodeficiency virus type 1; ITT-E=intention-to-treat exposed; INI=integrase inhibitor; LA=long-acting; mITT-E=modified intention-to-treat exposed; NNRTI=non-nucleoside reverse transcriptase inhibitor; RNA=ribonucleic acid.
REKAMBYS (rilpivirine long-acting injection), including the trademark, is owned by the Janssen Pharmaceutical Companies and used under licence by the ViiV Healthcare group of companies. All other trademarks are owned by the ViiV Healthcare group.
PM-GB-CBR-WCNT-230001 | March 2024
Adverse events should be reported. Reporting forms and information can be found at https://yellowcard.mhra.gov.uk/ or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GSK via the GSK Reporting Tool or on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.