THE REAL-WORLD INSIGHTS OF PEOPLE LIVING WITH HIV SHARED THROUGH ELECTRONIC DEVICES (RISE) STUDY
Background
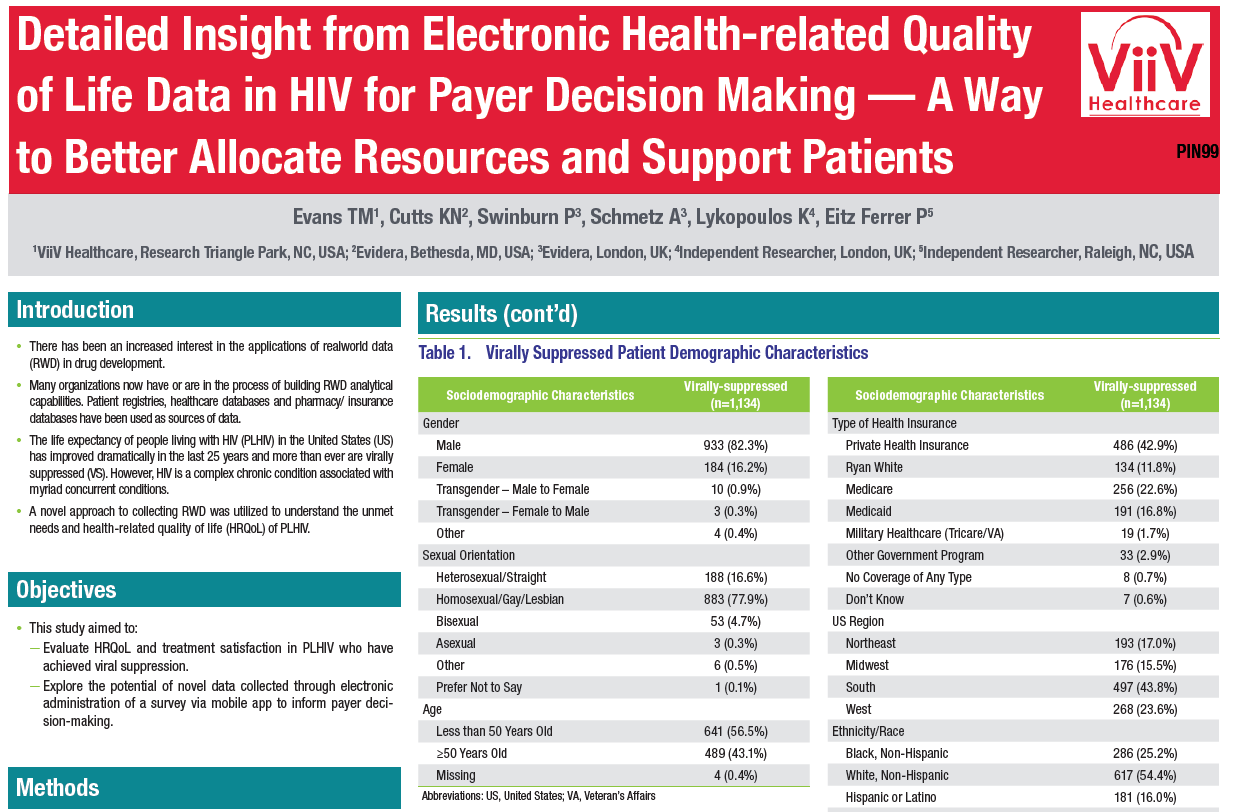
The “Real-world Insights of PLHIV Shared through Electronic Devices” (RISE) study was a cross-sectional survey designed to obtain an up-to-date understanding of the impact living with HIV has on patients’ lives in the US. This study aims to obtain a current understanding of the impact HIV has on patients’ lives in the United States and to evaluate health related quality of life, internalized stigma, and general satisfaction in PLHIV who have achieved viral suppression.


Design
A total of 1226 participants who were at least 21 years old with a self-reported diagnosis of HIV were included in the study. Additionally, all participants possessed a smartphone with internet access and were able to read English or Spanish. As part of the cross-sectional survey design, participants completed the RISE survey on a customized mobile application downloaded directly to their mobile device.
The study intervention flow included an initial outreach phase with a study introduction, four screening questions, and electronic consent. After completing initial outreach, participants downloaded the RISE study app via Apple or Android App Stores. Lastly, participants completed RISE study questionnaires via the app. The survey included a sociodemographic and/clinical form, and 7 validated patient-reported outcome measures (i.e. adherence, HIV-SDM). An additional qualitative assessment of PLHIV’s amenability to using the online application platform for PRO data collection is also being conducted.
Primary Objective
- Explore the potential of a novel PRO data collection method using electronic administration of a survey via mobile phone app
Conclusions
- Payers believe that RWD offers enormous potential for decision-making, especially in resource allocation or policy and formulary design.
- HIV patients still have unmet needs related to health-related quality of life that require consideration from a variety of stakeholders, including education and access to treatment to improve adherence.
- Electronic mobile patient surveys can be beneficial to inform decision-making in a wide range of diseases, including diabetes, asthma, and oncology.
Collaborators
Evidera (CRO)
Setting
Online
Location
U.S.A.
Duration
Jan 2018 – May 2021
Category
Health Related Quality of Life
Key study materials
RELATED STUDIES
USA
The Implementation Science team worked to evaluate the patient experience in the MORE program and evaluate MORE’s impact on ART adherence among HIV positive medical patients of Whitman-Walker Health who have inconsistent attendance to appointments.
USA
To aid future research on the effectiveness of the U=U message campaign as a clinical intervention, the aim of this project is to evaluate the effectiveness of a clinic-delivered media campaign of the U=U message.
USA
This study aims to determine the reach of the U=U message and better understand the context for implementation across different audiences using social media and other virtual approaches implemented by community ambassadors.
Study focus areas
Our implementation research projects have a global reach and focus on improving the HIV prevention and care continuum.

NP-GBL-HVX-WCNT-220049 October 2023
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.