PREDICTING HSR
Before initiating treatment with abacavir
Screening for HLA-B*5701 should be performed.
Abacavir should never be initiated in patients with a positive HLA-B*5701 status, nor in patients with a negative HLA-B*5701 status who had a suspected abacavir HSR on a previous abacavir-containing regimen.
HLA-B*5701 testing must not be used as a diagnostic test after a patient has started treatment with abacavir
Clinical diagnosis of suspected hypersensitivity to abacavir remains the basis for clinical decision making.
The role of HLA-B*5701
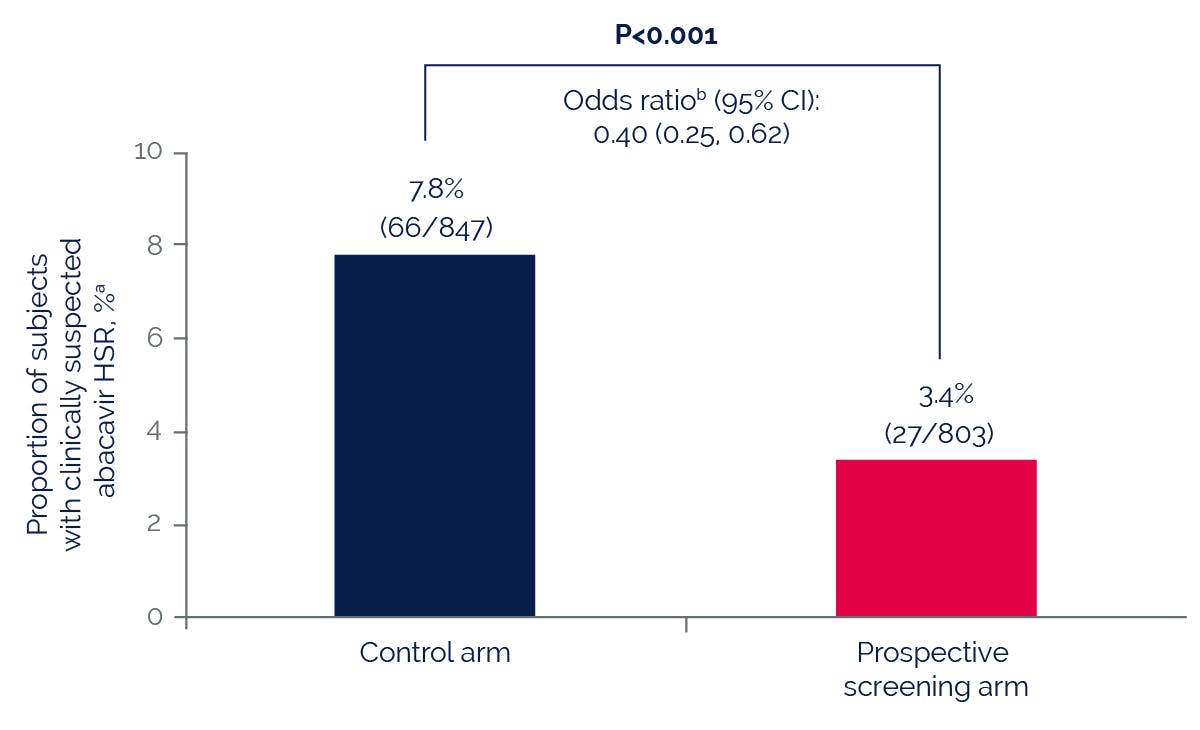
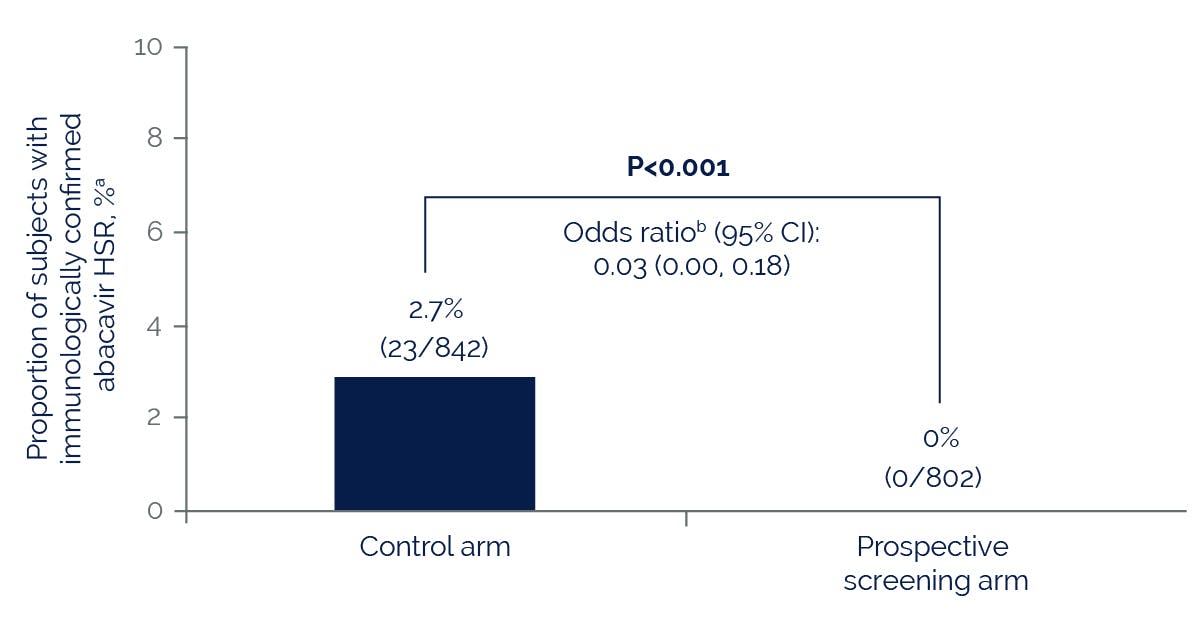
The HLA-B*5701 allele is more common among patients who have a suspected hypersensitivity reaction to abacavir compared with those who do not, regardless of race.
No other pharmacogenetic markers have been found that identify patients at risk of abacavir hypersensitivity.
Prospective pharmacogenetic screening for HLA-B*5701 can be used to identify patients at high risk for abacavir hypersensitivity.
However, HLA-B*5701 is not always present in people who have a suspected abacavir HSR. Therefore, clinical diagnosis of suspected HSR to abacavir remains the basis of clinical decision making.
HLA-B*5701 screening for risk of abacavir HSR should never be substituted for appropriate clinical vigilance and patient management in individuals receiving abacavir.
HLA-B*5701 screening
Before initiating treatment with abacavir, screening for HLA-B*5701 should be performed.
Screening is also recommended prior to re-initiation of abacavir in patients of unknown HLA-B*5701 status who have previously tolerated abacavir.
HLA-B*5701 status must always be documented and explained to the patient prior to initiating therapy.
In HLA-B*5701–negative patients, clinical vigilance remains vital to detect any abacavir hypersensitivity at the earliest stage.
Results of pharmacogenetic tests for risk of abacavir hypersensitivity should never be used to support a drug rechallenge decision after a suspected hypersensitivity reaction.
HLA-B*5701 testing must not be used as a diagnostic test after a patient has started treatment with abacavir.
NP-GBL-HVX-WCNT-220048 September 2024
Adverse events should be reported. Reporting forms and information can be found at yellowcard.mhra.gov.uk or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.