DDIS BETWEEN RECOMMENDED AND ALTERNATIVE EACS INITIAL REGIMENS AVAILABLE AS A SINGLE PILL AND 43 COMMONLY PRESCRIBED NON-ARVS AS DEFINED BY EACS*[1]
DOVATO HAS FEWER ANTICIPATED DDIs THAN OTHER EACS-RECOMMENDED SINGLE PILL REGIMENS
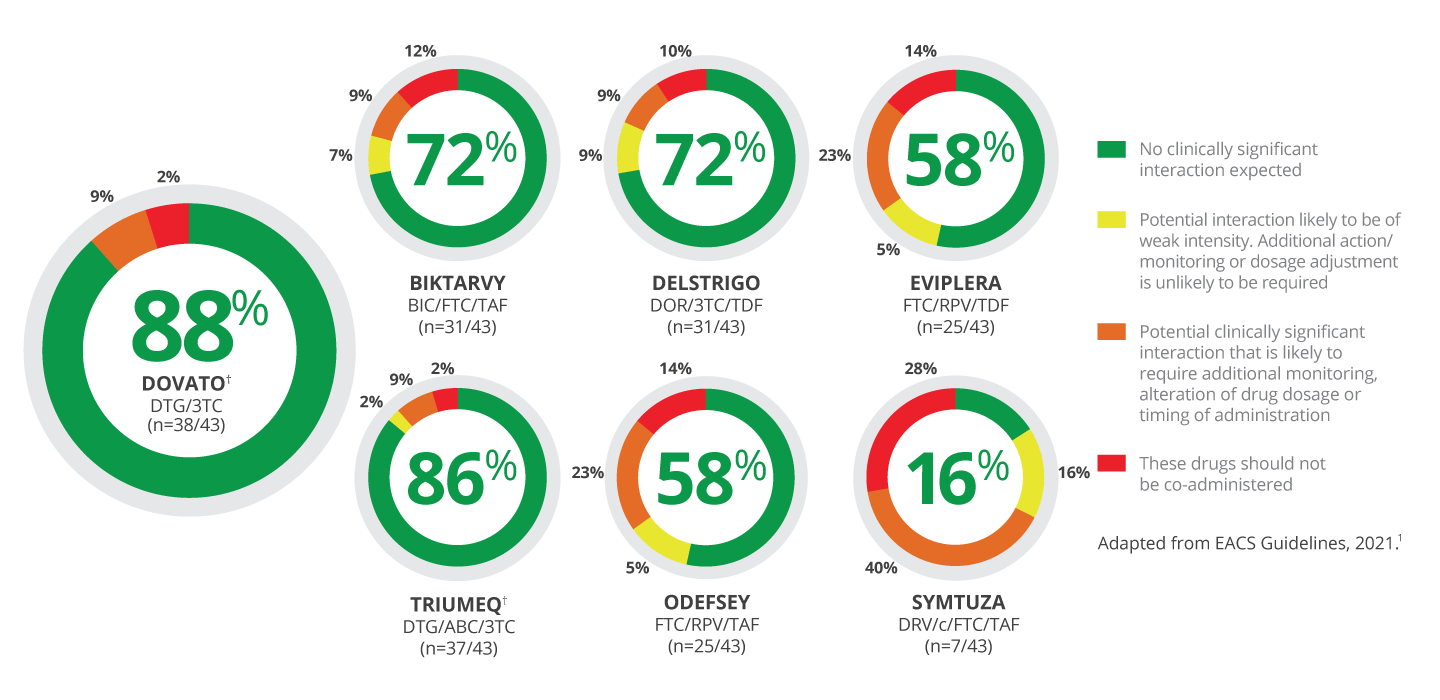
*Includes 43 non-ARVs across cardiovascular drugs, CNS drugs, anti-infectives and miscellaneous (eg, antacids, PPIs, H2 blockers, methadone and St John’s wort). Included in EACS Guidelines as commonly co-prescribed or of particular clinical relevance. Original data source: University of Liverpool HIV drug interaction data.[1]
†Contraindicated with fampridine.[2,3]
DOVATO DRUG-DRUG INTERACTIONS
- Minimal effect on metabolism via the CYP3A4 pathway
- No known interactions with contraceptives, antihypertensives, proton pump inhibitors, statins, PDE-5 inhibitors or recreational drugs
- Contraindication with substrates of OCT-2 with narrow therapeutic windows, such as fampridine
- Avoid chronic co-administration of sorbitol and similar solutions
- The combination of Dovato with cladribine is not recommended
- A dose adjustment of metformin should be considered when starting and stopping co‑administration of Dovato, to maintain glycaemic control. In patients with moderate renal impairment a dose adjustment of metformin should be considered when co-administered with Dovato, because of the increased risk for lactic acidosis in patients with moderate renal impairment due to increased metformin concentration
- The recommended dose of dolutegravir is 50 mg twice daily when co-administered with rifampicin, oxcarbazepine, carbamazepine, phenytoin, phenobarbital, St John’s Wort, etravirine (without boosted protease inhibitors), efavirenz, nevirapine, tipranavir/ritonavir. As Dovato is a fixed-dose tablet, an additional 50 mg tablet of dolutegravir should be administered approximately 12 hours after Dovato for the duration of co-administration (a separate formulation of dolutegravir is available for this dose adjustment)
- Polyvalent cation-containing antacids are recommended to be taken 2 hours after or 6 hours before Dovato and should not be co-administered at the same time
- Supplements or multivitamins containing calcium, iron or magnesium can be taken at the same time as Dovato when with food. When Dovato is taken without food, it is recommended that the supplement is taken 2 hours after or 6 hours before Dovato
Please see Dovato SmPC for full DDI information before prescribing Dovato
References:
- European AIDS Clinical Society. Guidelines. Version 11.0. October 2021. Accessed December 17,
2021. https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf - DOVATO (dolutegravir/lamivudine) Summary of Product Characteristics.
- TRIUMEQ (dolutegravir/ abacavir/ lamivudine) Summary of Product Characteristics.
July 2024 PM-GB-DLL-WCNT-220003 v2
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.


