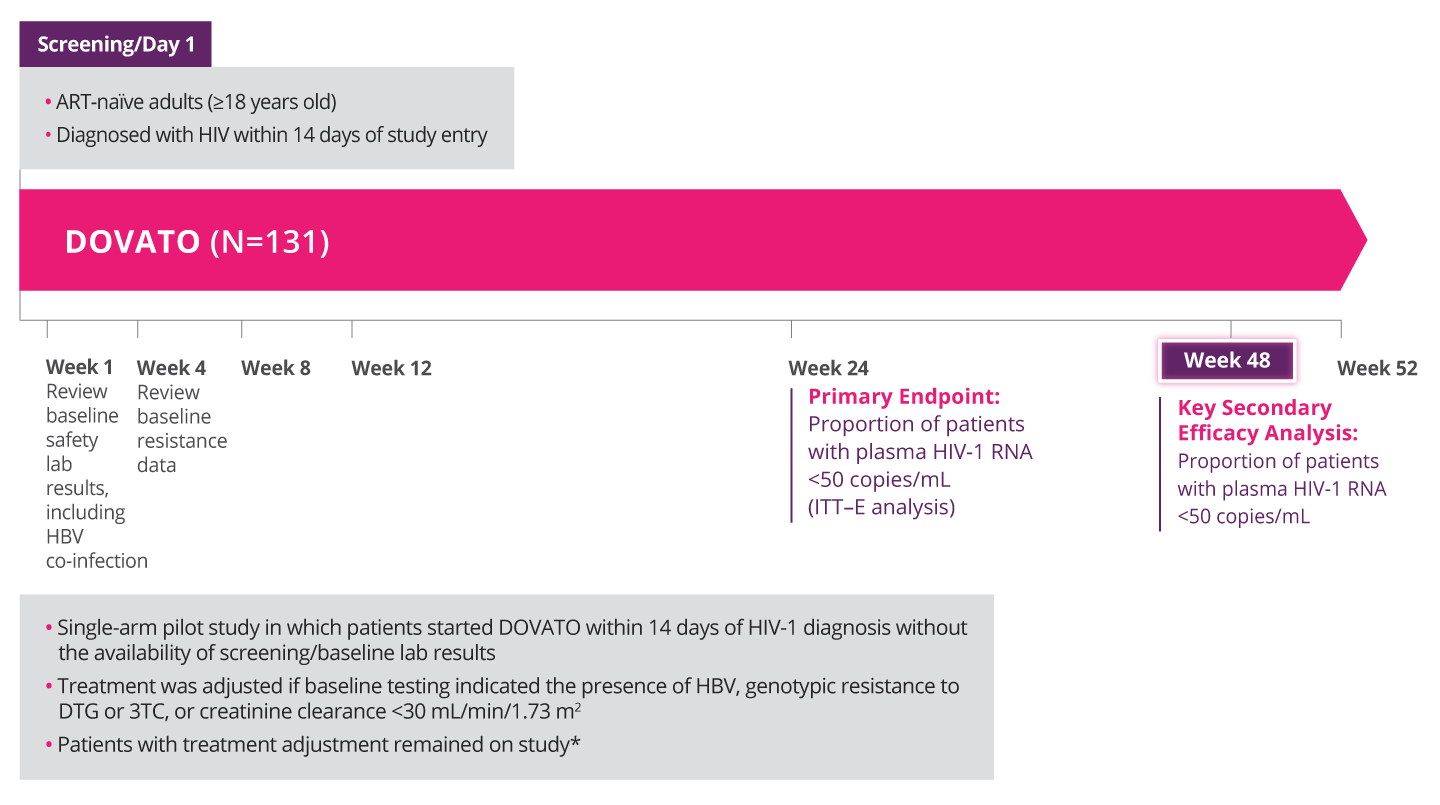
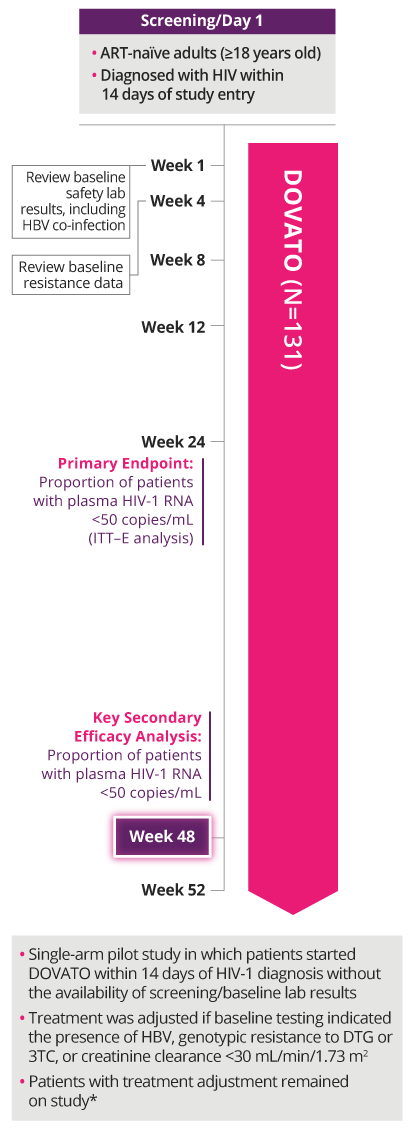
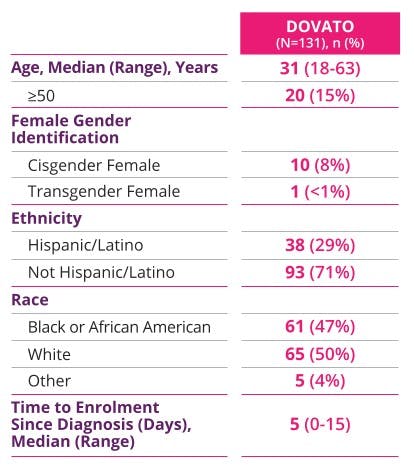
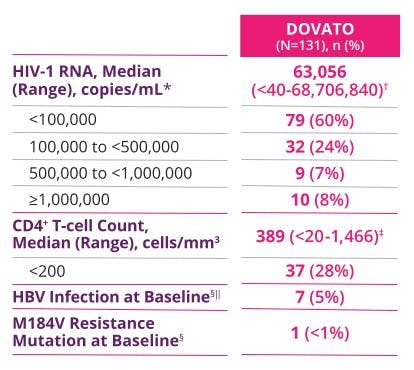
PROVEN EFFICACY IN A TEST-AND-TREAT SETTING
Virological Outcomes at Week 48[1]


Adapted from Rolle et al, 2021.[1]
ITT–E=intent-to-treat–exposed.
*Proportion of participants with plasma HIV-1 RNA <50 copies/mL, regardless of ART regimen, among those with available HIV-1 RNA at Week 48.
†Proportion of all participants with plasma HIV-1 RNA <50 copies/mL at Week 48, regardless of ART regimen.
‡Proportion of all participants with plasma HIV-1 RNA <50 copies/mL at Week 48 still taking DOVATO.
Summary of Virological Outcomes at Week 48[2]


Adapted from Rolle et al, 2021.[2]
ROBUST EFFICACY, EVEN IN PLHIV WITH >1 MILLION copies/mL
Virological Outcomes by Baseline Viral Load or CD4+ T-cell Count at 48 Weeks (ITT–E Missing=Failure Analysis; Accounts for Patients Who May Have Switched Regimens)[1]


Adapted from Rolle et al, 2021.[1]
ITT–E=intent-to-treat–exposed.
*One (<1%) participant had missing plasma HIV-1 RNA results at baseline.
A TOLERABILITY PROFILE YOU EXPECT FROM DOVATO[1]


Adapted from Rolle et al, 2021.[1]
AE=adverse event; SAE=serious adverse event.
*All AEs were Grade 2.
†Two SAEs occurred (cellulitis, streptococcal bacteraemia). No fatal SAEs occurred.
‡All psychiatric AEs were Grade 1 or Grade 2. AEs were coded using MedDNA v23.1.

Want to hear from the experts, including past webinar recordings?
References:
- Rolle C-P, Berhe M, Singh T, et al. High results of virologic suppression with DTG/3TC in newly diagnosed adults with HIV-1 infection and baseline viral load ≥500,000 c/mL: 48-week subgroup analysis of the STAT study. Presented at: IDWeek 2021; September 29-October 3, 2021; Virtual.
- Rolle C-P, Berhe M, Singh T, et al. Feasibility, efficacy, and safety of dolutegravir/lamivudine (DTG/ 3TC) as a first-line regimen in a test-and-treat setting for newly diagnosed people living with HIV (PLWH): 48-week results of the STAT study. Presented at: The 11th International AIDS Society Conference on HIV Science; July 18-21, 2021; Virtual. Poster PEB182.
- Rolle C-P, Berhe M, Singh T, et al. Dolutegravir/lamivudine as a first-line regimen in a test-and-treat setting for newly diagnosed people living with HIV. AIDS. 2021;35(12):1957-1965. doi:10.1097/QAD.0000000000002979
October 2022 PM-GB-DLL-WCNT-220004
Adverse events should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard or search for MHRA Yellowcard in the Google Play or Apple App store. Adverse events should also be reported to GlaxoSmithKline on 0800 221441.
If you are from outside the UK, you can report adverse events to GSK/ViiV by selecting your region and market, here.